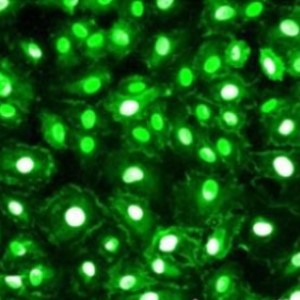
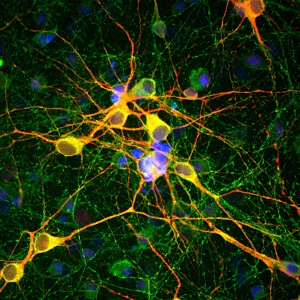
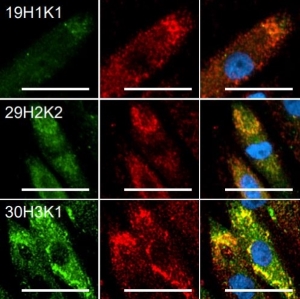
- Mu Opioid Receptor—Rabbit
- Kelsey Vollmer, Lisa Green, Roger Grant, Kion Winston, Elizabeth Doncheck, Christopher Bowen, Jacqueline Paniccia, Rachel Clarke, Annika Tiller, Preston Siegler, Benjamin Siemsen, Adam Denton, Thomas Jhou, Jennifer Rinker, Michael Scoeld, and James Otis. (2022). A Thalamostriatal Parvalbumin Interneuron Circuit Suppresses Risky Reward-Motivated Behaviors and Is Disengaged by Opioids. doi: 10.21203/rs.3.rs-1527325/v1
- Amanda Lillywhite, Stephen G. Woodhams, Sara V. Goncalves, David J.G. Watson, Li Li, James J. Burston, Peter R.W. Gowler,, Meritxell Canals, David A. Walsh, Gareth J. Hathway, Victoria Chapman. (2021). Anxiety Enhances Pain In a Model of Osteoarthritis and Is Associated with Altered Endogenous Opioid Function and Reduced Opioid Analgesia. Pain Reports. doi: 10.1097/PR9.0000000000000956
- Ching Chang, Hung-Kai Liu, Chao-Bin Yeh, Ming-Lin Yang, Wen-Chieh Liao Chiung-Hui Liu, and To-Jung Tseng. (2021). Cross-Talk of Toll-Like Receptor 5 and Mu-Opioid Receptor Attenuates Chronic Constriction Injury-Induced Mechanical Hyperalgesia through a Protein Kinase C Alpha-Dependent Signaling. International Journal of Molecular Sciences, 22(4), 189. doi: 10.3390/ijms22041891
- Yize Li, Xinying Guo, Linlin Sun, Jifang Xiao, Songxue Su, Shibin Du, Zhen Li, Shaogen Wu, Weili Liu, Kai Mo, Shangzhou Xia, Yun‐Juan Chang, Daniel Denis, and Yuan‐Xiang Tao. (2020). N6‐Methyladenosine Demethylase FTO Contributes to Neuropathic Pain by Stabilizing G9a Expression in Primary Sensory Neurons. Advanced Science. doi: 10.1002/advs.201902402
- Xiaqing Ma, Rui Chen, Min Huang, Wenying Wang, Limin Luo, Dong Kwan Kim, Wei Jiang, and Tao Xuab. (2020). DAMGO-Induced μ Opioid Receptor Internalization and Recycling Restore Morphine Sensitivity in Tolerant Rat. European Journal of Pharmacology. doi: 10.1016/j.ejphar.2020.173118
- Caroline Johnson, Weizhe Hong and Paul Micevych (2020). Optogenetic activation of β-endorphin terminals in the medial preoptic nucleus regulates sexual receptivity. eNeuro 15 January 2020, ENEURO.0315-19.2019; DOI: https://doi.org/10.1523/ENEURO.0315-19.2019
- Jie Sun, Shao-Rui Chen, Hui-Lin Pan (2020). μ-Opioid receptors in primary sensory neurons are involved in supraspinal opioid analgesia. Brain Research Volume 1729, https://doi.org/10.1016/j.brainres.2019.146623
- Batsheva Reich Yan Zhou Ellen Goldstein Sudarshan S. Srivats Natalina H. Contoreggi Joshua F. Kogan Bruce S. McEwen Mary Jeanne Kreek Teresa A. Milner Jason D. Gray (2019). Chronic immobilization stress primes the hippocampal opioid system for oxycodone associated learning in female but not male rats. Synapse. Accepted Author Manuscript. doi:10.1002/syn.22088
- Jie Sun Shao‐Rui Chen Hong Chen Hui‐Lin Pan. (2018). μ‐Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid‐induced hyperalgesia. The Journal of Physiology. https://doi.org/10.1113/JP277428
- Min-Ho Nam, Kyung-Seok Han, Jaekwang Lee, Jin Young Bae, Heeyoung An, Seahyung Park, Soo-Jin Oh, Eunju Kim, Eunmi Hwang, Yong Chul Bae, and C. Justin Lee. (2018). Expression of µ-Opioid Receptor in CA1 Hippocampal Astrocytes. Exp Neurobiol. 2018 Apr;27(2):120-128. English. Published online April 24, 2018. https://doi.org/10.5607/en.2018.27.2.120
- Fenghua Bao, Chang-Lin Li, Xu-Qiao Chen, Ying-Jin Lu, Lan Bao, and Xu Zhang. (2018). Clinical Opioids Differentially Induce Co-Internalization of μ- and δ-Opioid Receptors. Molecular Pain. doi: https://doi.org/10.1177/1744806918769492
- TJ Tseng, ML Yang, YL Hsieh, MH Ko, ST Hsieh. (2017). Nerve Decompression Improves Spinal Synaptic Plasticity of Opioid Receptors for Pain Relief. Neurotoxicity Research, doi: 10.1007/s12640-017-9799-5
- Wendy M. Walwyn, Wenling Chen, Hyeyoung Kim, Ani Minasyan, Helena S. Ennes, James A. McRoberts, and Juan Carlos G. Marvizón. (2016). Sustained Suppression of Hyperalgesia during Latent Sensitization by μ-, δ-, and κ-opioid receptors and α2A Adrenergic Receptors: Role of Constitutive Activity. The Journal of Neuroscience, 36(1): 204-221. doi: 10.1523/JNEUROSCI.1751-15.2016
- Sherika N. Smith, Candler Paige, Kandy T. Velazquez, Terika P. Smith, Srinivasa N. Raja, Steven P. Wilson, Sarah M. Sweitzer. (2015). Injury-specific promoters enhance herpes simplex virus mediated gene therapy for treating neuropathic pain in rodents. The Journal of Pain, doi: 10.1016/j.jpain.2014.12.007
- Fiona B Carr, Sandrine M Géranton and Stephen P Hunt. (2014). Descending controls modulate inflammatory joint pain and regulate CXC chemokine and iNOS expression in the dorsal horn. Molecular Pain, 10:39. doi: 10.1186/1744-8069-10-39
- Shafaq Sikandar, Kirsty Bannister, Anthony H. Dickenson. (2012). Brainstem facilitations and descending serotonergic controls contribute to visceral nociception but not pregabalin analgesia in rats. Neuroscience Letters, doi: 10.1016/j.neulet.2012.05.009
- Andrew C. Eschenroeder, Allison A. Vestal-Laborde, Emilse S. Sanchez, Susan E. Robinson, Carmen Sato-Bigbee. (2012). Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of μ-opioid- and nociceptin/orphanin FQ receptors in cell development: Implications for drug addiction treatment during pregnancy. Glia, Volume 60, Issue 1, pages 125–136. doi: 10.1002/glia.21253
- De Felice, Milena, Sanoja, Raul, Wang, Ruizhong, Vera-Portocarrero, Louis, Oyarzo, Janice, King, Tamara, Ossipov, Michael H., Porreca, Frank. (2011). Engagement of descending inhibition from the rostral ventromedial medulla protects against chronic neuropathic pain. PAIN, 152 (12), p.2701-2709. doi: 10.1016/j.pain.2011.06.008
- Ryan J. Horvathab, Alfonso Romero-Sandoval, Joyce A. De Leo. (2010). Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and μ opioid receptor protein expression while enhancing perivascular microglial ED2. PAIN, Pages 401-413. doi: 10.1016/j.pain.2010.02.042
- Hai-Bo Wanga, Bo Zhaoa, Yan-Qing Zhonga, Kai-Cheng Li, Zi-Yan Li, Qiong Wang, Yin-Jing Lua, Zhen-Ning Zhang, Shao-Qiu He, Han-Cheng Zheng, Sheng-Xi Wu, Tomas G. M. Hökfelt, Lan Baob, and Xu Zhanga. (2010). Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. PNAS, vol. 107 no. 29 13117-13122. doi: 10.1073/pnas.1008382107
- Marta Pinto, Marta Sousa, Deolinda Lima, Isaura Tavares. (2008). Participation of mu-opioid, GABAB, and NK1 receptors of major pain control medullary areas in pathways targeting the rat spinal cord: Implications for descending modulation of nociceptive transmission. The Journal of Comparative Neurology, Volume 510, Issue 2, Pages 175 - 187. doi: 10.1002/cne.21793
- Mu Opioid Receptor—Guinea Pig
- Yingfu Jiao, Po Gao, Li Dong, Xiaowei Ding, Youqiang Meng, Jiahong Qian, Ting Gao, Ruoxi Wang, Tao Jiang, Yunchun Zhang, Dexu Kong, Yi Wu, Sihan Chen, Saihong Xu, Dan Tang, Ping Luo, Meimei Wu, Li Meng, Daxiang Wen, Changhao Wu, Guohua Zhang, Xueyin Shi, Weifeng Yu, and Weifang Rong. (2022). Molecular Identification of Bulbospinal ON Neurons by GPER Which Drives Pain And Morphine Tolerance. The Journal of Clinical Investigation.
- Weifang Rong, Ting Gao, Li Dong, Jiahong Qian, Xiaowei Ding, Yi Zheng, Meimei Wu, Li Meng, Yingfu Jiao, Po Gao, Ping Luo, Guohua Zhang, Changhao Wu, and Xueyin Shi. (2021). G-Protein-Coupled Estrogen Receptor (GPER) in the Rostral Ventromedial Medulla Is Essential for Mobilizing Descending Inhibition of Itch. Journal of Neuroscience, 41(37), 7727-7741. doi: 10.1523/JNEUROSCI.2592-20.2021
- Gloria Brunori, Oliver B Pelletier, Anna M Stauffer, and Janet D Robishaw. (2021). Selective Manipulation of G-Protein γ7 Subunit in Mice Provides New Insights into Striatal Control of Motor Behavior. Journal of Neuroscience, 1211-21. doi: 10.1523/JNEUROSCI.1211-21.2021
- Akila Ram. (2021). The Periaqueductal Grey in Opioid Tolerance and Chronic Pain. Utah State University, Doctoral Dissertation
- Ting Zhang, Run Zhang, Biao Xu, Mengna Zhang, Qinqin Zhang, Ning Li, Yu Qiu, Dan Chen, Kangtai Xu, Jian Xiao, Nan Zhang, Quan Fang. (2021). Spinal Endomorphins Attenuate Burn-Injury Pain in Male Mice by Inhibiting p38 MAPK Signaling Pathway Through the Mu-Opioid Receptor. European Journal of Pharmacology, 174139. doi: 10.1016/j.ejphar.2021.174139
- Ting Zhang, Weidong Zhao, Mengna Zhang, Biao Xu, Xuerui Shia, Qinqin Zhang, Yuanyuan Guo, Jian Xiao, Dan Chen, Ting Zheng, Quan Fang. (2018). Analgesic activities of the mixed opioid and NPFF receptors agonist DN-9 in a mouse model of formalin-induced orofacial inflammatory pain. Peptides. https://doi.org/10.1016/j.peptides.2018.10.010
- Kazuya Hagimoto, Saki Takami, Fujio Murakami, Yasuto Tanabe. (2016). Distinct Migratory Behaviors of Striosome and Matrix Cells Underlying the Mosaic Formation in the Developing Striatum. The Journal of Comparative Neurology, doi: 10.1002/cne.24096
- Ying-Biao Chen, Fen-Sheng Huang, Ban Fen, Jun-Bin Yin, Wei Wang and Yun-Qing. (2015). Inhibitory effects of endomorphin-2 on excitatory synaptic transmission and the neuronal excitability of sacral parasympathetic preganglionic neurons in young rat. Frontiers in Cellular Neuroscience, doi: 10.3389/fncel.2015.00206
- J. Desroches, J.-F. Bouchard, L. Gendron, P. Beaulieu. (2014). Involvement of cannabinoid receptors in peripheral and spinal morphine analgesia. Neuroscience, Volume 261, Pages 23–42. doi: 10.1016/j.neuroscience.2013.12.030
- Shao-Qiu Hea, Fei Yanga, Federico M. Perezb, Qian Xua, Ronen Shechtera, Yong-Kwan Cheong, Alene F. Carteret, Xinzhong Dong, Sarah M. Sweitzer, Srinivasa N. Raja, Yun Guan. (2013). Tolerance to the anti-allodynic effects of the peripherally acting opioid, loperamide hydrochloride, develops in nerve-injured rats. PAIN, doi: 10.1016/j.pain.2013.07.023
- Rounak Nassirpour, Laia Bahima, Arnaud L. Lalive, Christian Lüscher, Rafael Luján, and Paul A. Slesinger. (2010). Morphine- and CaMKII-Dependent Enhancement of GIRK Channel Signaling in Hippocampal Neurons. J. Neurosci., Oct 2010; 30: 13419 - 13430. doi: 10.1523/JNEUROSCI.2966-10.2010
- Tara A. Macey, Janet D. Lowe, and Charles Chavkin. (2006). Mu Opioid Receptor Activation of ERK1/2 Is GRK3 and Arrestin Dependent in Striatal Neurons. J Biol Chem, doi: 10.1074/jbc.M60427820
Product Patent Application
- G. Scherrer, V. Tawfik, G. Corder. (2018). Methods for Attenuating or Preventing Mu-Opioid Receptor Mediated Tolerance and Opioid-Induced Hyperalgesia. United States Patent Application 20180214442.
- Delta Opioid Receptor 3-17—Rabbit
- Sophia C. Levis, Matthew T. Birnie, Yiyan Xie, Noriko Kamei, Puja V. Kulkarni, Johanna S. Montesinos, Christina R. Perrone, Catherine M. Cahill, Tallie Z. Baram, and Stephen V. Mahler. (2024). Opioid Drug Seeking After Early-Life Adversity: A Role for Delta Opioid Receptors. Addiction Neuroscience, 100175. doi: 10.1016/j.addicn.2024.100175
- Hai-Bo Wanga, Bo Zhaoa, Yan-Qing Zhonga, Kai-Cheng Li, Zi-Yan Li, Qiong Wang, Yin-Jing Lua, Zhen-Ning Zhang, Shao-Qiu He, Han-Cheng Zheng, Sheng-Xi Wu, Tomas G. M. Hökfelt, Lan Baob, and Xu Zhanga. (2010). Coexpression of δ- and μ-opioid receptors in nociceptive sensory neurons. PNAS, vol. 107 no. 29 13117-13122. doi: 10.1073/pnas.1008382107
- Delta Opioid Receptor 358-372—Rabbit
- Liping Xu, Josef Vagner, Jatinder Josan, Ronald M. Lynch, David L. Morse, Brenda Baggett, Haiyong Han, Eugene A. Mash, Victor J. Hruby and Robert J. Gillies. (2009). Enhanced targeting with heterobivalent ligands. Mol Cancer Ther, doi: 10.1158/1535-7163
- More Publications
- Edmund Wing Ong. (2017). Investigating the Effects of Prolonged Mu Opioid Receptor Activation Upon Opioid Receptor Heteromerization. Queen's University.
- Nathan Long, Bertha Long, Asma Mana, Dream Le, Lam Nguyen, Sima Chokr, Kevin Sinchak. (2017). Tamoxifen and ICI 182,780 activate hypothalamic G protein-coupled estrogen receptor 1 to rapidly facilitate lordosis in female rats. Hormones and Behavior, doi: 10.1016/j.yhbeh.2016.12.013
- Wen-Ling Dai, Feng Xiong, Bing Yan, Zheng-Yu Cao, Wen-Tao Liu, Ji-Hua Liu1, Bo-Yang Yu. (2016). Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord. Scientific Reports 6, Article number: 38746. doi: 10.1038/srep38746
- K. Lutfy, D. Parikh, D.L. Lee, Y. Liu, M.G. Ferrini, A. Hamid, T.C. Friedman. (2016). Prohormone convertase 2 (PC2) null mice have increased mu opioid receptor levels accompanied by enhanced morphine-induced antinociception, tolerance and dependence. Neuroscience, doi: 10.1016/j.neuroscience.2016.05.021
- Jan Fraessdorf, Markus W. Hollmann, Iris Hanschmann, André Heinen, Nina C. Weber, Benedikt Preckel, Ragnar Huhn. (2015). Role of Endogenous Opioid System in Ischemic-Induced Late Preconditioning. PLOS ONE, doi: 10.1371/journal.pone.0134283
- Joanna Mika, Katarzyna Popiolek-Barczyk, Ewelina Rojewska, Wioletta Makuch, Katarzyna Starowicz, Barbara Przewlocka. (2014). Delta-Opioid Receptor Analgesia Is Independent of Microglial Activation in a Rat Model of Neuropathic Pain. PLOS ONE, doi: 10.1371/journal.pone.0104420
- Xue-Long Zhou, Li-Na Yu1, Yin Wang, Li-Hui Tang, Yu-Nan Peng, Jun-Li Cao and Min Yan. (2014). Increased methylation of the MOR gene proximal promoter in primary sensory neurons plays a crucial role in the decreased analgesic effect of opioids in neuropathic pain. Molecular Pain, 10:51. doi: 10.1186/1744-8069-10-51
- Charlie H.T. Kwok, Ian M. Devonshire, Andrew J. Bennett, Gareth J. Hathway. (2014). Postnatal maturation of endogenous opioid systems within the periaqueductal grey and spinal dorsal horn of the rat. PAIN, Vol. 155, Issue 1, Pages 168-178. doi: 10.1016/j.pain.2013.09.0220
- Shao-Qiu Hea, Fei Yanga, Federico M. Perezb, Qian Xua, Ronen Shechtera, Yong-Kwan Cheong, Alene F. Carteret, Xinzhong Dong, Sarah M. Sweitzer, Srinivasa N. Raja, Yun Guan. (2013). Tolerance to the anti-allodynic effects of the peripherally acting opioid, loperamide hydrochloride, develops in nerve-injured rats. PAIN, doi: 10.1016/j.pain.2013.07.023
- Groer, Chad E, Cullen L. Schmid, Alex M. Jaeger and Laura M. Bohn. (2011). AGONIST-DIRECTED INTERACTIONS WITH SPECIFIC βARRESTINS DETERMINE MU OPIOID RECEPTOR TRAFFICKING, UBIQUITINATION, AND DEPHOSPHORYALTION. Journal of Biological Chemistry, doi: 10.1074/jbc.M111.248310
- Dominika Labuz, Yvonne Schmidt, Anja Schreiter, Heike L. Rittner, Shaaban A. Mousa and Halina Machelska. (2009). Immune cell–derived opioids protect against neuropathic pain in mice. J. Clin. Invest., doi: 10.1172/JCI36246
- Phoebe Dewing, Amy Christensen, Galyna Bondar and Paul Micevych. (2008). PKC signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology, doi: 10.1210/en.2008-0847
- Sasaki, Atsushi a; Nakashima, Yasutaka a; Takasaki, Ichiro c; Andoh, Tsugunobu a; Shiraki, Kimiyasu b; Kuraishi, Yasushi a d. (2008). Morphine inhibits herpetic allodynia through [mu]-opioid receptors induced in A[beta]-fiber neurons. Neuroreport, 19(9):975-979. doi: 10.1097/WNR.0b013e328302f123
- Zhang, Guohua M.D., Ph.D.; Mohammad, Husam; Peper, Brad D.; Raja, Srinivasa M.D; Wilson, Steven P. Ph.D; Sweitzer, Sarah M. Ph.D. (2008). Enhanced Peripheral Analgesia Using Virally Mediated Gene Transfer of the [mu]-Opioid Receptor in Mice. Anesthesiology, Volume 108, Issue 2. doi: 10.1097/01.anes.0000299836.61785.79
- Phoebe Dewing, Marissa I. Boulware, Kevin Sinchak, Amy Christensen, Paul G. Mermelstein, and Paul Micevych. (2007). Membrane Estrogen Receptor- Interactions with Metabotropic Glutamate Receptor 1a Modulate Female Sexual Receptivity in Rats. The Journal of Neuroscience, doi: 10.1523/JNEUROSCI.0592-07.2007
- S.-R. Chen, A. Prunean, H.-M. Pan, K.L. Welker and H.-L. Pan. (2007). Resistance to morphine analgesic tolerance in rats with deleted transient receptor potential vanilloid type 1-expressing sensory neurons. Neuroscience, doi: 10.1016/j.neuroscience.2006.12.016
- Eunhae Kim, Amy L. Clark , Alexi Kiss, Jason W. Hahn, Robin Wesselschmidt. (2006). MU AND KAPPA OPIOIDS INDUCE THE DIFFERENTIATION OF EMBRYONIC STEM CELLS TO NEURAL PROGENITORS JBC Papers in Press, doi: 10.1074/jbc.M603862200
- Thomas F. Finnegan, Shao-Rui Chen, and Hui-Lin Pan. (2005). Effect of the µ Opioid on Excitatory and Inhibitory Synaptic Inputs to Periaqueductal Gray-Projecting Neurons in the Amygdala. JPET, 312:441-448. doi: 10.1124/jpet.104.074633
- Shuxing Wang, Grewo Lim, Liling Yang, Qing Zeng, Backil Sung, J.A. Jeevendra Martyn and Jianren Mao. (2005). A rat model of unilateral hindpaw burn injury: Slowly developing rightwards shift of the morphine dose–response curve. Pain, doi: 10.1016/j.pain.2005..03.044
- Wu Z, Chen S and Pan H. (2004). Differential Sensitivity of N- and P/Q-Type Ca2+ Channel Currents to a µ Opioid in Isolectin B -Positive and -Negative Dorsal Root Ganglion Neurons. JPET, 311:939-947. doi: 10.1124/jpet.104.073429
- Chen S and Pan H. (2003). Antinociceptive Effect of Morphine, but not Mu Opioid Receptor Number, Is Attenuated in the Spinal Cord of Diabetic Rats. Anesthesiology, 99:1409–14.
- Jones T, Sweitzer S, Wilson S, and Yeomans D. (2003). Afferent fiber-selective shift in opiate potency following targeted opioid receptor knockdown. Pain, 106, 365–371. doi: 10.1016/j.pain.2003.08.006


Blog Posts