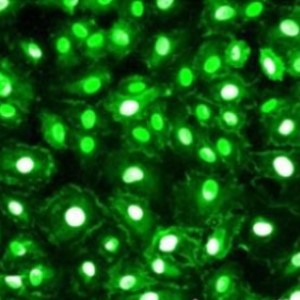
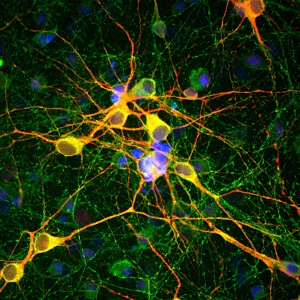
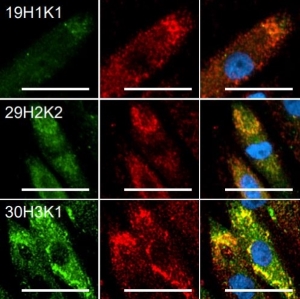
- Antigen-Specific Cell Isolation
Strep-Tactin® PE (Cat. 6-5000-001)
- J. Maximilian Fels, et al. (2021). Protective Neutralizing Antibodies from Human Survivors of Crimean-Congo Hemorrhagic Fever. Cell, 184(13), 3486-3501.E21. doi: 10.1016/j.cell.2021.05.001
- Benjamin Faist, et al. (2019). Targeted in-vitro-Stimulation Reveals Highly Proliferative Multi-Virus-Specific Human Central Memory T Cells As Candidates for Prophylactic T Cell Therapy. PLoS ONE, 14(9): e0223258. doi: 10.1371/journal.pone.0223258
- Fabian Schlott, et al. (2018). Characterization and Clinical Enrichment of HLA-C*07:02-Restricted Cytomegalovirus-Specific CD8+ T Cells. Plos One. doi: 10.1371/journal.pone.0193554
- Monika Fröhlich, et al. (2016). Interrupting CD28 Costimulation Before Antigen Rechallenge Affects CD8+ T-Cell Expansion and Effector Functions During Secondary Response in Mice. European Journal of Immunology, 46(7), 1644-1655. doi: 10.1002/eji.201546232
- M. Cioni, et al. (2016). Characterization of Immunodominant BK Polyomavirus 9mer Epitope T Cell Responses. American Journal of Transplantation, 16(4), 1193-1206. doi: 10.1111/ajt.13598
- C S Link, et al. (2016). Abundant Cytomegalovirus (CMV) Reactive Clonotypes in the CD8+ T Cell Receptor Alpha Repertoire Following Allogeneic Transplantation. Clinical and Experimental Immunology, 184(3), 389–402. doi: 10.1111/cei.12770
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
Strep-Tactin® APC (Cat. 6-5010-001)
- J. Maximilian Fels, et al. (2021). Protective Neutralizing Antibodies from Human Survivors of Crimean-Congo Hemorrhagic Fever. Cell, 184(13), 3486-3501.E21. doi: 10.1016/j.cell.2021.05.001
- Benjamin Faist, et al. (2019). Targeted in-vitro-Stimulation Reveals Highly Proliferative Multi-Virus-Specific Human Central Memory T Cells As Candidates for Prophylactic T Cell Therapy. PLoS ONE, 14(9): e0223258. doi: 10.1371/journal.pone.0223258
- Fabian Mohr, et al. (2018). Efficient Immunoaffinity Chromatography of Lymphocytes Directly from Whole Blood. Scientific Reports, 8: 16731. doi: 10.1038/s41598-018-34589-z
- Fabian Schlott, et al. (2018). Characterization and Clinical Enrichment of HLA-C*07:02-Restricted Cytomegalovirus-Specific CD8+ T Cells. Plos One. doi: 10.1371/journal.pone.0193554
- Magdalena Nauerth, et al. (2016). Flow Cytometry-Based TCR-Ligand Koff-Rate Assay for Fast Avidity Screening of Even Very Small Antigen-Specific T Cell Populations ex vivo. Cytometry, 89(9), 816-825. doi: 10.1002/cyto.a.22933
CD81 Fab-Strep, human (Cat. 6-8015-150)
- Ondřej Pelák, et al. (2016). Lymphocyte Enrichment Using CD81-Targeted Immunoaffinity Matrix. Cytometry, 91(1), 62-72. doi: 10.1002/cyto.a.22918
MHC I-Strep HLA-A*0201; CMV pp65 (NLVPMVATV) (Cat. 6-7001-001)
- M Raeiszadeh, et al. (2006). The T Cell Response to Persistent Herpes Virus Infections in Common Variable Immunodeficiency. Clinical and Experimental Immunology, 146(2), 234–242, doi: 10.1111/j.1365-2249.2006.03209.x
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
MHC I-Strep H-2 Kb; Ovalbumin (SIINFEKL) (Cat. 6-7015-001)
- Patricia Graef, et al. (2014). Serial Transfer of Single-Cell-Derived Immunocompetence Reveals Stemness of CD8+ Central Memory T Cells. Immunity, 41(1), 116-126. doi: 10.1016/j.immuni.2014.05.018
- Christian Stemberger, et al. (2007). A Single Naive CD8+ T Cell Precursor Can Develop into Diverse Effector and Memory Subsets. Immunity, 27(6), 985-997. doi: 10.1016/j.immuni.2007.10.012
MHC I-Strep HLA-A*0101; CMV pp50 (VTEHDTLLY) (Cat. 6-7024-001)
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
MHC I-Strep HLA-B*0702; CMV pp65 (TPRVTGGGAM) (Cat. 6-7027-001)
- Özcan Çınar, et al. (2021). High-Affinity T-Cell Receptor Specific for MyD88 L265P Mutation for Adoptive T-Cell Therapy of B-Cell Malignancies. Journal for ImmunoTherapy of Cancer, 9, e002410. doi: 10.1136/jitc-2021-002410
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
MHC I-Strep HLA-A*2402; CMV pp65 (QYDPVAALF) (Cat. 6-7028-001)
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
MHC I-Strep HLA-A*0201; CMV IE-1 (VLEETSVML) (Cat. 6-7041-001)
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
MHC I-Strep H-2 Db; LCMV gp (SGVENPGGYCL) (Cat. 6-7097-001)
- Juliana Bessa, et al. (2007). Efficient Induction of Mucosal and Systemic Immune Responses by Virus-Like Particles Administered Intranasally: Implications for Vaccine Design. European Journal of Immunology, 38(1), 114-126. doi: 10.1002/eji.200636959
MHC I-Strep HLA-A*0201; WT1 (VLDFAPPGA) (Cat. 6-7099-001)
- Xinchao Wang, et al. (2010). Streptamer-Based Selection of WT1-Specific CD8+ T Cells for Specific Donor Lymphocyte Infusions. Immunotherapy, 38(11), 1066-1073. doi: 10.1016/j.exphem.2010.07.002
More Examples
- Miriam Ciáurriz, et al. (2016). Streptamer Technology Allows Accurate and Specific Detection of CMV-Specific HLA-A*02 CD8+ T Cells by Flow Cytometry. Clinical Cytometry, 92(2), 153-160. doi: 10.1002/cyto.b.21367
- Christian Stemberger, et al. (2014). Lowest Numbers of Primary CD8+ T Cells Can Reconstitute Protective Immunity Upon Adoptive Immunotherapy. Blood, 124(4), 628–637. doi: 10.1182/blood-2013-12-547349
- Xiaoqian Wang, et al. (2008). Dynamics of Proximal Signaling Events after TCR/CD8-Mediated Induction of Proliferation or Apoptosis in Mature CD8+ T Cells. Journal of Immunology, 180(10), 6703–6712. doi: 10.4049/jimmunol.180.10.6703
- Marc Dauer, et al. (2006). IFN-α Promotes Definitive Maturation of Dendritic Cells Generated by Short-Term Culture of Monocytes with GM-CSF and IL-4. Journal of Leukocyte Biology, 80(2), 278–286. doi: 10.1189/jlb.1005592
- Cell Isolation & Staining
Strep-Tactin® PE (Cat. 6-5000-001)
- J. Maximilian Fels, et al. (2021). Protective Neutralizing Antibodies from Human Survivors of Crimean-Congo Hemorrhagic Fever. Cell, 184(13), 3486-3501.E21. doi: 10.1016/j.cell.2021.05.001
- Benjamin Faist, et al. (2019). Targeted in-vitro-Stimulation Reveals Highly Proliferative Multi-Virus-Specific Human Central Memory T Cells As Candidates for Prophylactic T Cell Therapy. PLoS ONE, 14(9): e0223258. doi: 10.1371/journal.pone.0223258
- Fabian Schlott, et al. (2018). Characterization and Clinical Enrichment of HLA-C*07:02-Restricted Cytomegalovirus-Specific CD8+ T Cells. Plos One. doi: 10.1371/journal.pone.0193554
- Monika Fröhlich, et al. (2016). Interrupting CD28 Costimulation Before Antigen Rechallenge Affects CD8+ T-Cell Expansion and Effector Functions During Secondary Response in Mice. European Journal of Immunology, 46(7), 1644-1655. doi: 10.1002/eji.201546232
- M. Cioni, et al. (2016). Characterization of Immunodominant BK Polyomavirus 9mer Epitope T Cell Responses. American Journal of Transplantation, 16(4), 1193-1206. doi: 10.1111/ajt.13598
- C S Link, et al. (2016). Abundant Cytomegalovirus (CMV) Reactive Clonotypes in the CD8+ T Cell Receptor Alpha Repertoire Following Allogeneic Transplantation. Clinical and Experimental Immunology, 184(3), 389–402. doi: 10.1111/cei.12770
- Marcus Odendahl, et al. (2014). Clinical-Scale Isolation of ‘Minimally Manipulated’ Cytomegalovirus-Specific Donor Lymphocytes for the Treatment of Refractory Cytomegalovirus Disease. Cytotherapy, 16(9), 1245-1256, doi: 10.1016/j.jcyt.2014.05.023
Strep-Tactin® APC (Cat. 6-5010-001)
- J. Maximilian Fels, et al. (2021). Protective Neutralizing Antibodies from Human Survivors of Crimean-Congo Hemorrhagic Fever. Cell, 184(13), 3486-3501.E21. doi: 10.1016/j.cell.2021.05.001
- Benjamin Faist, et al. (2019). Targeted in-vitro-Stimulation Reveals Highly Proliferative Multi-Virus-Specific Human Central Memory T Cells As Candidates for Prophylactic T Cell Therapy. PLoS ONE, 14(9): e0223258. doi: 10.1371/journal.pone.0223258
- Fabian Mohr, et al. (2018). Efficient Immunoaffinity Chromatography of Lymphocytes Directly from Whole Blood. Scientific Reports, 8: 16731. doi: 10.1038/s41598-018-34589-z
- Fabian Schlott, et al. (2018). Characterization and Clinical Enrichment of HLA-C*07:02-Restricted Cytomegalovirus-Specific CD8+ T Cells. Plos One. doi: 10.1371/journal.pone.0193554
- Magdalena Nauerth, et al. (2016). Flow Cytometry-Based TCR-Ligand Koff-Rate Assay for Fast Avidity Screening of Even Very Small Antigen-Specific T Cell Populations ex vivo. Cytometry, 89(9), 816-825. doi: 10.1002/cyto.a.22933
Strep-Tactin® Magnetic Microbeads (Cat. 6-5510-050)
- Sarah Matko, et al. (2017). Enumeration of WT1-Specific CD8+ T Cells for Clinical Application Using an MHC Streptamer Based No-Wash Single-Platform Flow-Cytometric Assay. Cytometry Part A, 91(10), 1001-1008. doi: 10.1002/cyto.a.23
CD3 Fab-Strep, Human (Cat. 6-8001-150)
- Benjamin Faist, et al. (2019). Targeted in-vitro-Stimulation Reveals Highly Proliferative Multi-Virus-Specific Human Central Memory T Cells As Candidates for Prophylactic T Cell Therapy. PLoS ONE, 14(9): e0223258. doi: 10.1371/journal.pone.0223258
CD3 Fab-TACS® Agarose Column Starter Kit, Human (Cat. 6-3201-002)
- Philipp Boosz, et al. (2021). Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles Enable a Stable Non-Spilling Loading of T Cells and Their Magnetic Accumulation. Cancers, 13(16), 4143. doi: 10.3390/cancers13164143
- M Mühlberger, et al. (2019). Functionalization of T Lymphocytes With Citrate-Coated Superparamagnetic Iron Oxide Nanoparticles For Magnetically Controlled Immune Therapy. International Journal of Nanomedicine, 14:8421-8432. doi: 10.2147/IJN.S218488
CD8 Fab-TACS® Agarose Column Starter Kit, Human (Cat. 6-3203-002)
- Immanuel Andra, et al. (2020). An Evaluation of T-Cell Functionality After Flow Cytometry Sorting Revealed p38 MAPK Activation. Cytometry, 97(2), 171-183. doi: 10.1002/cyto.a.23964
- Cell Stimulation & Expansion
CD3/CD8 Streptamer® Kit, Human (Cat. 6-8900-050)
- Liying An, et al. (2022). Concurrent Stimulation of Monocytes with CSF1 and Polarizing Cytokines Reveals Phenotypic and Functional Differences with Classical Polarized Macrophages. Journal of Leukocyte Biology, 112(3), 437-447. doi: 10.1002/JLB.3A0721-383R
- Fabian Mohr, et al. (2018). Efficient Immunoaffinity Chromatography of Lymphocytes Directly from Whole Blood. Scientific Reports, 8: 16731. doi: 10.1038/s41598-018-34589-z
- Sarah Matko, et al. (2017). Enumeration of WT1-Specific CD8+ T Cells for Clinical Application Using an MHC Streptamer Based No-Wash Single-Platform Flow-Cytometric Assay. Cytometry Part A, 91(10), 1001-1008. doi: 10.1002/cyto.a.23146
CMV pp65 peptide NLVPMVATV (HLA-A*0201) (Cat. 6-7001-901)
- Sarah Matko, et al. (2017). Enumeration of WT1-Specific CD8+ T Cells for Clinical Application Using an MHC Streptamer Based No-Wash Single-Platform Flow-Cytometric Assay. Cytometry Part A, 91(10), 1001-1008. doi: 10.1002/cyto.a.23146
- Junxia Yao, et al. (2008). Multimer Staining of Cytomegalovirus Phosphoprotein 65–Specific T Cells for Diagnosis and Therapeutic Purposes: A Comparative Study. Clinical Infectious Diseases, 46(10), e96–e105. doi: 10.1086/587749
WT1 peptide RMFPNAPYL (HLA-A*0201) (Cat. 6-7019-901)
- Sarah Matko, et al. (2017). Enumeration of WT1-Specific CD8+ T Cells for Clinical Application Using an MHC Streptamer Based No-Wash Single-Platform Flow-Cytometric Assay. Cytometry Part A, 91(10), 1001-1008. doi: 10.1002/cyto.a.23146
CMV pp65 peptide TPRVTGGGAM (HLA-B*0702) (Cat. 6-7027-901)
- Sarah Matko, et al. (2017). Enumeration of WT1-Specific CD8+ T Cells for Clinical Application Using an MHC Streptamer Based No-Wash Single-Platform Flow-Cytometric Assay. Cytometry Part A, 91(10), 1001-1008. doi: 10.1002/cyto.a.23146
- Junxia Yao, et al. (2008). Multimer Staining of Cytomegalovirus Phosphoprotein 65–Specific T Cells for Diagnosis and Therapeutic Purposes: A Comparative Study. Clinical Infectious Diseases, 46(10), e96–e105. doi: 10.1086/587749
More Examples
- More References
- Ting Jiang, et al. (2021). 5-Aza-2-deoxycytidine Alleviates the Progression of Primary Biliary Cholangitis by Suppressing the FoxP3 Methylation and Promoting the Treg/Th17 Balance. International Immunopharmacology, 96, 107820. doi: 10.1016/j.intimp.2021.107820
- Yaara Finkel, et al. (2021). SARS-CoV-2 Uses a Multipronged Strategy to Impede Host Protein Synthesis. Nature, 594, 240–245. doi: 10.1038/s41586-021-03610-3
- Emanuele Andreano, et al. (2021). Extremely Potent Human Monoclonal Antibodies from COVID-19 Convalescent Patients. Cell, 184, 1821–1835. doi: 10.1016/j.cell.2021.02.035
- Emma C. Thomson, et al. (2021). Circulating SARS-CoV-2 Spike N439K Variants Maintain Fitness while Evading Antibody-Mediated Immunity. Cell, 184(5), 1171-1187.E20. doi: 10.1016/j.cell.2021.01.037
- Bing Rao, et al. (2021). The Cryo-EM Structure of an ERAD Protein Channel Formed by Tetrameric Human Derlin-1. Science Advances, 7(10). doi: 10.1126/sciadv.abe8591
- Miguel de Almeida Fuzeta, et al. (2019). Addressing the Manufacturing Challenges of Cell-Based Therapies. Current Applications of Pharmaceutical Biotechnology, 171. doi: 10.1007/10_2019_118
- Eleanor M. Denham, et al. (2019). A Generic Cell Surface Ligand System for Studying Cell–Cell Recognition. PLoS Biology, 17(12): e3000549. doi: 10.1371/journal.pbio.3000549
- Christophe J. Lalaurie, et al. (2018). The de novo Design of a Biocompatible and Functional Integral Membrane Protein Using Minimal Sequence Complexity. Scientific Reports, 8, 14564. doi: 10.1038/s41598-018-31964-8
- Marina Mühlberger, et al. (2019). Non-Magnetic Chromatographic Separation of Colloidally Metastable Superparamagnetic Iron Oxide Nanoparticles and Suspension Cells. Journal of Chromatography B, 1122–1123, 83-89. doi: 10.1016/j.jchromb.2019.05.033
- Marthe C.J. Roex, et al. (2018). The Simultaneous Isolation of Multiple High and Low Frequent T-Cell Populations from Donor Peripheral Blood Mononuclear Cells Using the Major Histocompatibility Complex I-Streptamer Isolation Technology. Cytotherapy, 20(4), 543-555. doi: 10.1016/j.jcyt.2018.01.008
- Yorke Zhang, et al. (2017). A Semi-Synthetic Organism that Stores and Retrieves Increased Genetic Information. Nature, 551, 644–647. doi: 10.1038/nature24659
- Fabian Mohr, et al. (2017). Minimally Manipulated Murine Regulatory T Cells Purified by Reversible Fab Multimers are Potent Suppressors for Adoptive T-Cell Therapy. European Journal of Immunology, 47(12), 2153-2162. doi: 10.1002/eji.201747137
- Aurore Saudemont, et al. (2017). Immunotherapy after Hematopoietic Stem Cell Transplantation Using Umbilical Cord Blood-Derived Products. Cancer Immunology, Immunotherapy, 66, 215–221. doi: 10.1007/s00262-016-1852-3
- Catherine M. Bollard, et al. (2016). T Cells for Viral Infections After Allogeneic Hematopoietic Stem Cell Transplant. Blood, 127(26), 3331–3340. doi: 10.1182/blood-2016-01-628982
- Shu-Qi Zhang, et al. (2016). Direct Measurement of T Cell Receptor Affinity and Sequence from Naïve Antiviral T Cells. Science Translational Medicine, 8(341), 341ra77. doi: 10.1126/scitranslmed.aaf1278
- Enrico Maffini, et al. (2016). Treatment of CMV Infection After Allogeneic Hematopoietic Stem Cell Transplantation. Expert Review of Hematology, 9(6), 585-596. doi: 10.1080/17474086.2016.1174571
- R J O'Reilly, et al. (2016). Virus-Specific T-Cell Banks for 'Off the Shelf' Adoptive Therapy of Refractory Infections. Bone Marrow Transplantation, 51, 1163–1172. doi: 10.1038/bmt.2016.17
- Swait Naik, et al. (2016). Adoptive Immunotherapy for Primary Immunodeficiency Disorders with Virus-Specific T Lymphocytes. Immune Deficiencies, Infection, and Systemic Immune Disorders, 137(5), 1498-1505. doi: 10.1016/j.jaci.2015.12.1311
- Xiuyan Wang, et al. (2016). Clinical Manufacturing of CAR T Cells: Foundation of a Promising Therapy. Molecular Therapy Oncolytics, 3, 16015. doi: 10.1038/mto.2016.15
- Sander Kelderman, et al. (2016). Antigen-Specific TIL Therapy for Melanoma: A Flexible Platform for Personalized Cancer Immunotherapy. European Journal of Immunology, 46(6), 1351-1360. doi: 10.1002/eji.201646436
- Keenan T Bashour, et al. (2015). Functional Characterization of a T Cell Stimulation Reagent for the Production of Therapeutic Chimeric Antigen Receptor T Cells. Blood, 126(23), 1901. doi: 10.1182/blood.V126.23.1901.1901
- Luca Gattinoni. (2014). Memory T Cells Officially Join the Stem Cell Club. Immunity, 41(1), 7-9. doi: 10.1016/j.immuni.2014.07.003
- Michael Schmitt, et al. (2014). Peptide Vaccines for Patients with Acute Myeloid Leukemia. Expert Review of Vaccines, 8(10), 1415-1425. doi: 10.1586/erv.09.90
- Bianca Weissbrich, et al. (2013). Adoptive Immunotherapy. OncoImmunology, 2(10), e26199. doi: 10.4161/onci.26199
- Magdalena Nauerth, et al. (2013). TCR-Ligand koff Rate Correlates with the Protective Capacity of Antigen-Specific CD8+ T Cells for Adoptive Transfer. Science Translational Medicines, 5(192), 192ra87, doi: 10.1126/scitranslmed.3005958
- Xin-Chao Wang, et al. (2013). Streptamer Versus Tetramer-Based Selection of Functional Cytomegalovirus-Specific T Cells. Journal of the Formosan Medical Association, 112(6), 338-345. doi: 10.1016/j.jfma.2012.02.020
- Coen Govers, et al. (2012). Magnetic-Activated Cell Sorting of TCR-Engineered T Cells, Using tCD34 as a Gene Marker, but Not Peptide–MHC Multimers, Results in Significant Numbers of Functional CD4+ and CD8+ T Cells. Human Gene Therapy Methods, 23(3). doi: 10.1089/hgtb.2012.074
- Christian Stemberger, et al. (2012). Novel Serial Positive Enrichment Technology Enables Clinical Multiparameter Cell Sorting. PLoS ONE, 7(4): e35798. doi: 10.1371/journal.pone.0035798
- Anita Schmitt, et al. (2010). Adoptive Transfer and Selective Reconstitution of Streptamer-Selected Cytomegalovirus-Specific CD8+ T Cells Leads to Virus Clearance in Patients After Allogeneic Peripheral Blood Stem Cell Transplantation. Transfusion, 51(3), 591-599. doi: 10.1111/j.1537-2995.2010.02940.x
- Rosaely Casalegno-Garduño, et al. (2009). Multimer Technologies for Detection and Adoptive Transfer of Antigen-Specific T Cells. Cancer Immunology, Immunotherapy, 59, 195–202. doi: 10.1007/s00262-009-0778-4
- Christian Stemberger, et al. (2009). Stem Cell-Like Plasticity of Naïve and Distinct Memory CD8+ T Cell Subsets. Seminars in Immunology, 21(2), 62-68. doi: 10.1016/j.smim.2009.02.004
- Marc Dauer, et al. (2008). Combined Use of Toll-Like Receptor Agonists and Prostaglandin E2 in the FastDC Model: Rapid Generation of Human Monocyte-Derived Dendritic Cells Capable of Migration and IL-12p70 Production. Journal of Immunological Methods, 337(2), 97-105. doi: 10.1016/j.jim.2008.07.003
- C. Bauer, et al. (2008). Vaccination Therapy of Pancreatic Carcinoma Patients with Autologous, Tumor-Lysate Pulsed Dendritic Cells: Results of a Phase II Study. Journal of Clinical Oncology, 26(15), 3069-3069. doi: 10.1200/jco.2008.26.15_suppl.3069
- Julia Neudorfer, et al. (2007). Reversible HLA Multimers (Streptamers) for the Isolation of Human Cytotoxic T Lymphocytes Functionally Active Against Tumor- and Virus-Derived Antigens. Journal of Immunological Methods, 320(1–2), 119-131. doi: 10.1016/j.jim.2007.01.001
- Marc Dauer, et al. (2005). FastDC Derived from Human Monocytes within 48 h Effectively Prime Tumor Antigen-Specific Cytotoxic T Cells. Journal of Immunological Methods, 302(1–2), 145-155. doi: 10.1016/j.jim.2005.05.010
- R Munker, et al. (2004). An Update on Graft-Versus-Host and Graft-Versus-Leukemia Reactions: A Summary of the Sixth International Symposium Held in Schloss Ellmau, Germany, January 22–24, 2004. Bone Marrow Transplantation, 34, 767–780. doi: 10.1038/sj.bmt.1704667
- Michael Knabel, et al. (2002). Reversible MHC Multimer Staining for Functional Isolation of T-Cell Populations and Effective Adoptive Transfer. Nature Medicine, 8, 631–637. doi: 10.1038/nm0602-631
- Products
- Customer Publications
- Back
- Customer Publications
- Publications Using Our Human Primary Cells, iPSC Derived Cells & Cell Lines
- Publications Using Our Fetal Bovine Serum (FBS) & Other Sera
- Publications Using Our Defined Media & Culturing Tools
- Publications Using IBA Lifesciences Products
- Publications Using Our 3D in vivo Like Models
- Publications Using Our Antibodies
- Publications Using Our Proteins, Growth Factors & Enzymes
- Publications Using Our Vectors & Transfection Kits
- Protocols
- Testimonials
- Ordering Info & Policy
- Blog
- Back
- Blog
- Exciting Spinal Cord Injury Research
- Tumor Microenvironment Research Demands Our CAFs
- Cancer & Pain Intersect with Our Schwann Cells
- Surgical Imaging Research With Our Brain Cancer Cells
- Easy-To-Use RNase Inactivating Reagent
- Study the Tumor Microenvironment with Our CAFs
- Introducing Cas9 Expressing Primary Cells & GPCR Expressing Cell Lines
- Study Pain & Neuroscience with Our Antibodies
- 3D Bioprinting With Neuromics ISOKine Proteins
- More Diabetes Research With Our Endothelial Cells
- Impactful Neurodegenerative Disease Research
- Great Pricing on Reliable Human Cells
- CAFs Used in Groundbreaking Research
- FBS for $199/500 ml - Order Today
- Save 15% on All Human Cells - One Final Month
- Exciting Research Using Our HBMECs
- GFP Brain Cells in Surgery Research
- Save on IBA's Newest Resin This November
- Reliable Human Cell Lines
- More GFP-HUVECs Research
- Continue to Save on Neuromics Human Cells
- Don't Forget About Our GFP Expressing Cells
- Alzheimer's Research with Our 3D BBB Model
- Our Immortalized CAFs in Action
- Save Big on Human Cells to End The Year
- Rely On Our FBS For Fall Research
- Even More iPSC Brain Cells
- More Immortalized CAF Types
- IBA's New Protein Purification Resin
- Another Round of New iPSC Brain Cells
- Human Brain Cells That Make Discoveries
- Our HRMECs Enable Diabetes Research
- Save on Preferred FBS This Spring
- New Medias for Neurite Outgrowth & Tube Formation
- Cancer Associated Fibroblasts (CAFs) That Deliver
- More Research Releases Using Our Bio-Reagents
- Our FBS Continues to Impress
- Neuromics Products in Parkinson's Disease Research
- iPSC Brain Cells Available Today
- Introducing AlphaBioCoat Precoated Products
- End 2023 with FBS Savings
- Reliable FBS Makes Everything Easier
- More Blood-Brain Barrier Research
- Neuromics Primary Brain Cells in BBB Research
- Long COVID Research Uses Our Human Brain Cells
- Our Products Crush Pain & Cancer Research
- Study T-Cells with Our Peptides
- Endless Antibody Applications
- Versatile FBS Options
- Our HBMECs Used in Hypertension Research
- Welcoming Microglia to the Club
- Introducing More Cancer Associated Fibroblasts (CAFs)
- Another Pain Antibody Pub
- So Much FBS News to Share
- BBB Organoids Grown Using Neuromics Cells
- Research Proven Transfection Kits
- Pain Antibodies With 20+ Years of Proven Results
- Our Preferred FBS Is Back in Stock
- Two New FBS Publications to Share
- Fresh Antibodies for Fall Research
- New Research Using Our Human Brain Astrocytes
- More Savings This August
- Our Primary Human Neurons Enable Discoveries
- Save on FBS This July
- Another New Cell Type
- Free Shipping On FBS Through May
- A Number of Versatile Research Tools
- New Fibroblasts To Complement Our CAFs
- 5% Off Premium FBS Through the End of March
- A Collection of Products for Cancer Researchers
- Our Neurons Help Investigate the COVID-19 Virus
- Neuromics HBMECs and FBS Need More Attention
- So Many Reagents for Neuroscientists
- The Complete Set of Human Prostate Cancer Cell Lines
- Neuromics FBS Wins on Quality & Price
- Introducing Preferred FBS From Neuromics
- Out of Matrigel? Check Out Our Collagel Hydrogels
- Save on Antibodies While They Last
- Neuromics Brain Endothelial Cells Strike Again
- A Great Cell Line for Prostate Cancer Research
- Save This Month on Human Cells
- Our Antibodies Are Crushing Neuroscience Research
- Congrats to SSFC on a Great Season
- Neuromics HRMECs Prove Their Consistency
- Our Reagents are Helping Answer Questions About COVID-19
- New Peptides for COVID-19 Research
- Welcome to July... And FBS Savings!
- New Fibroblast Cells and More Research
- Study SARS-CoV2 and More with Our 3D BBB Model
- It's Summer: Save on Antibodies
- Neuromics Cells and Diabetes Research
- FBS and Serum June Update
- SSFC Has Started Off Strong
- FBS and Serum May Update
- New Brain Cancer Cells
- Our Sunflower's Are Back in Action
- Neuromics FBS and Cancer Research
- Our Tuj-1 Antibody Is the Real Deal
- The Sunflower's are Doing Awesome Things
- Neuromics Colorectal Tumor CAFs Are Helping Us Understand Cancer
- Neuromics is Celebrating Brain Awareness Week
- An Explanation of Our FBS Products
- FBS Still on Sale
- Our Human Endothelial Cells Are Elite
- Culture ALL Cell Types with Our FBS
- Premium Imported FBS Only $299!
- You'll Love Our Pericytes and Astrocytes
- Our Human Schwann Cells Walk the Talk
- Neuromics and Sunflower State FC
- Neuromics' Cells Used in COVID-19 Research
- New ACE2-GFP Human Cells
- Neuromics’ Colorectal Tumor and Pancreatic-Stellate Cell CAFs in Action
- Neuromics FBS Used in New Neurodegenerative Research
- New Publication Using Our Transfection Kit
- Save on Chicken Serum with Neuromics
- New Human Brain Cells
- COVID-19 is passing through the Blood-Brain Barrier!
- New PGP 9.5 Publications
- GFAP Publications
- More New Antibodies
- Save On Our FBS
- Calbindin Antibodies
- Blood-Brain Barrier Publication
- Lung Cancer Associated Fibroblasts Publications
- New Tumor Cells
- Blood-Brain Barrier Antibodies
- New Cluster of Differentiation (CD) Antibodies
- New Antibodies to Explore
- Your Data - Our Products
- Energize Your Cell-Based Assays
- Fibroblast Compression and Tumor Cells Migration
- Human Brain Cells
- Easy Immunostaining Staining
- New Neuronal Markers
- ATP and Pain
- Our Hearing Adapts in Space
- Staning Cells and Tissue
- Markers for Tyrosine Hydroxylase (TH)
- Human Cells in Action
- Neuromics' Fetal Bovine Serum (FBS) Strikes Again
- You'll Love Our FBS
- New Antibodies
- Your Opinion Matters
- May News
- HUVECS in 3-D Action
- Antibodies You Can Trust
- Long-Term Cell Cultures
- ABCA1, ASIC3 and MOR
- National Eye Institute's 3-D ROC Challenge
- April News
- Contact Us
- About Us
- FAQs
- Sign In
Blog Posts