Comparing Avi-tag and Twin-Strep-tag® in Surface Plasmon Resonance (SPR) Applications
Surface plasmon resonance (SPR) is an innovative technique to study biomolecular interactions. It allows the analysis of the affinity and kinetics of a specific protein to other proteins, small molecules, DNA, RNA, or cells. The technique is often used in protein analysis as one of the final steps in protein characterization.
Immobilization of the ligand to the biosensor chip is a crucial step. Both are made possible using an affinity tag.
Different affinity tags for immobilization
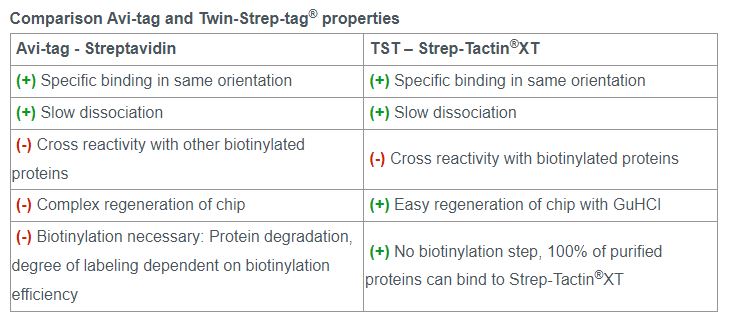
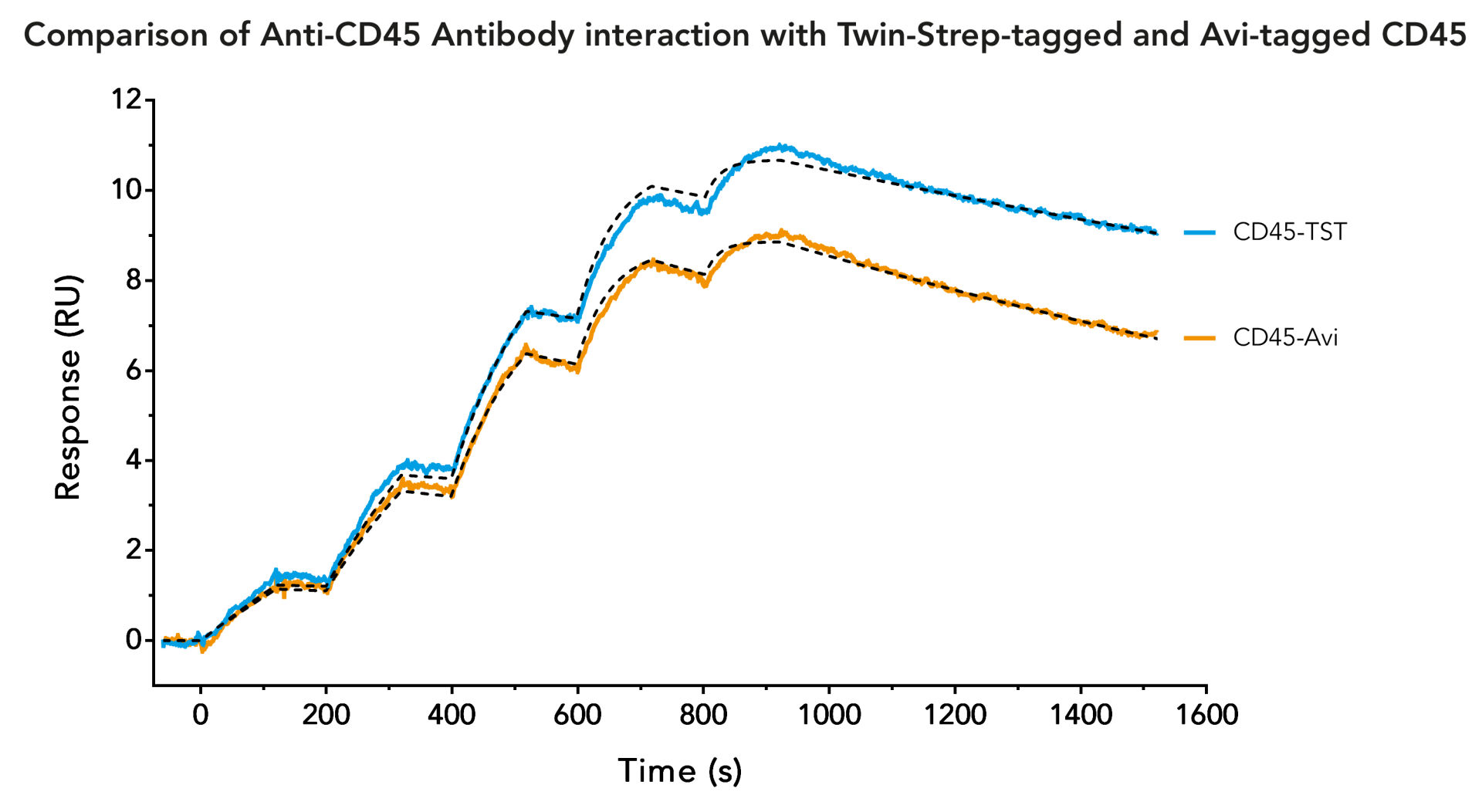
In SPR analysis, the Avi-tag is commonly used due to the high affinity and specificity of biotin to its ligand streptavidin. The Twin-Strep-tag® and Strep-Tactin®XT system is based on the same principle and delivers similar results as the Avi-tag system. However, the Twin-Strep-tag® has additional advantages not only in SPR, but as a universal tag for different applications during the protein analysis workflow.
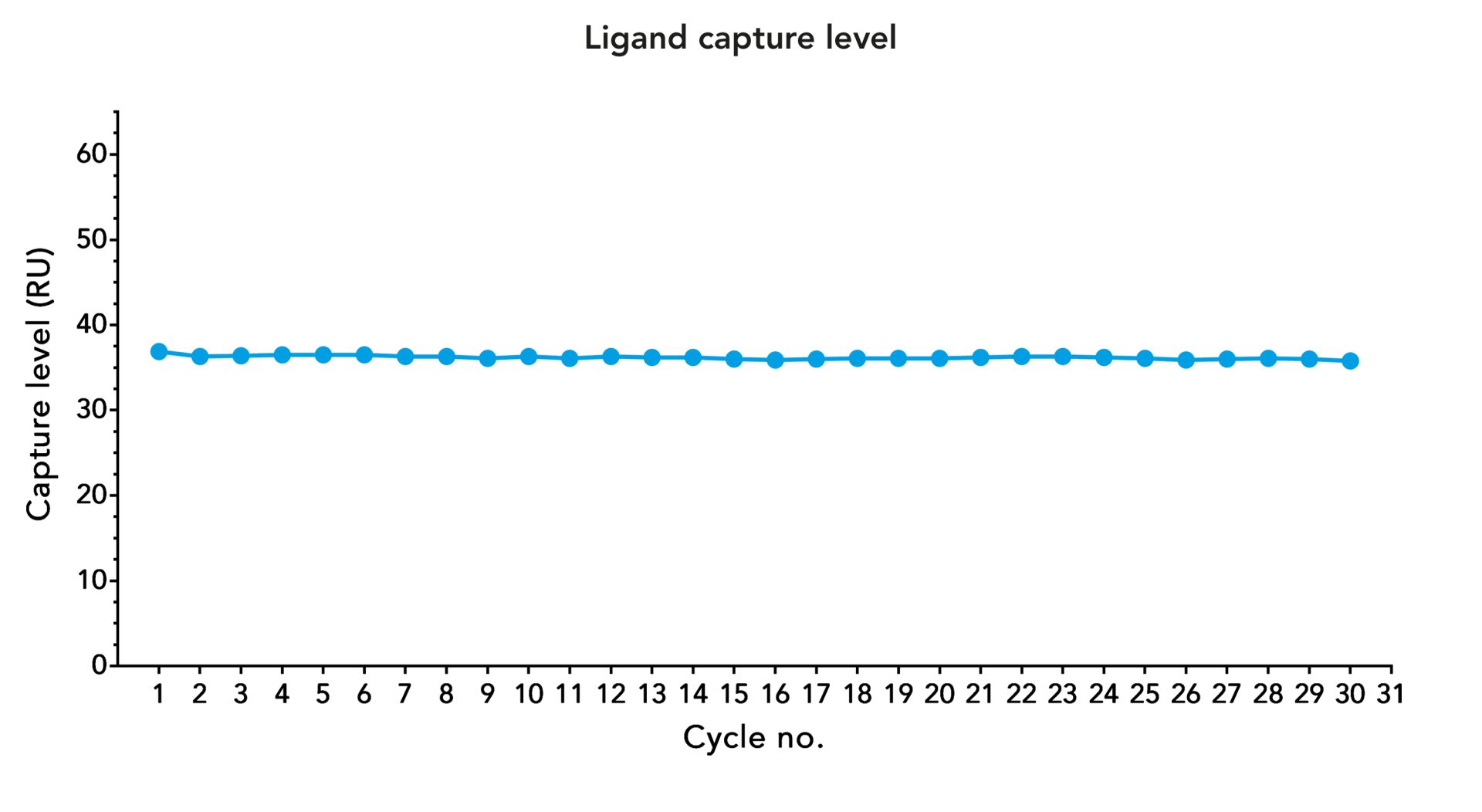
Strep-Tactin®XT is compatible with many reagents. This allows the use of flexible buffer systems during purification and analysis. Furthermore, Strep-Tactin®XT coated sensor chips can easily be regenerated while the capture level stays consistent over several regeneration cycles.
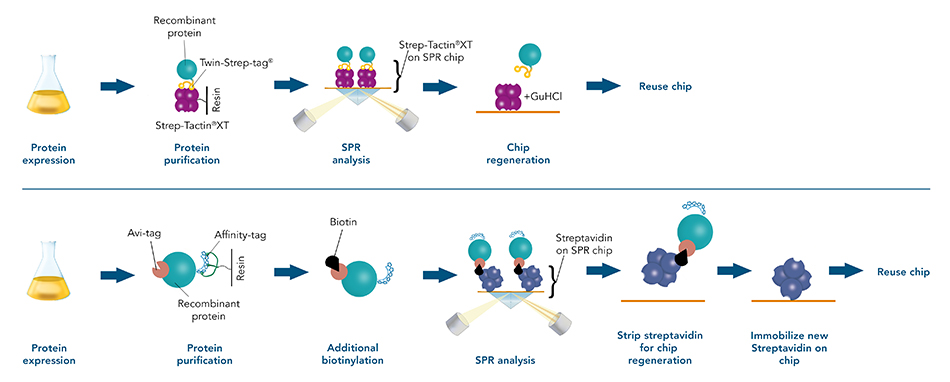
Comparison of the Twin-Strep-tag® and the Avi-tag protein analysis workflow
Both the Twin-Strep-tag® and the Strep-Tactin®XT ligand have been optimized to enable a strong bond that can be reversed by adding biotin. The Avi-tag binding is not reversible, which limits its usability for target protein purification.
The Twin-Strep-tag®/Strep-Tactin®XT system can be used for both purification and analysis of the protein of interest. As a result, SPR analysis can be seamlessly performed after the purification.