Exosomes have an endosomal origin and are produced by almost every cell type. Their size ranges between 30 and 150 nanometers in diameter and thus they belong to the smallest extracellular vesicles. Exosomes possess surface proteins that partly originate from plasma membranes during endocytosis. The tetraspanins CD9, CD63 and CD81 are specially enriched on the surface of these vesicles and are often used as biomarkers.
Exosomes transfer specific biomolecules such as DNA, RNA, proteins, enzymes as well as lipids and thereby mediate cell-to-cell communication. After their release, they carry information to neighboring or distant cells. This intercellular vesicle traffic allows rapid and controlled communication and therefore plays an important role in many aspects of health and disease, including development, immunity, tissue homeostasis, cancer, and neurodegenerative diseases.

Exosomes have become an attractive research target during the last years, as they participate in different physiological and/or pathological processes. They were identified as a promising clinical treatment option due to their immunomodulatory and anti-inflammatory properties. They have been linked to eliciting innate and adaptive immune responses, which potentially helps the fight against infectious agents or cancer and supports their utility for therapy development. In fact, exosome-based therapies may hold some advantages over cell-based therapies as they avoid many risks associated with cell transplantations.
Highly pure and functional exosome populations are especially required, when studying exosomes and their functions. However, due to the small size of exosomes that partly overlaps with other extracellular vesicles such as apoptotic bodies (100 – 5000 nm) or microparticles (100 - 1000 nm), their isolation remains challenging.
Virtually all cells release exosomes, which are most commonly identified by the tetraspanins CD9, CD81 and CD63 on their surface. Exosomes carry different molecules such as proteins, RNA or DNA and mediate cell-to-cell communication.
Exosome isolation methods
Commonly used exosome isolation methods include differential centrifugation, size exclusion chromatography, filtration, and PEG (polyethylene glycol (PEG)ylation). The basic principle of these methods is to separate the exosomes from other components in the sample based on particle size and/or physical properties.
However, these methods are very complex and time-consuming and often result in the purification of larger vesicles, impure vesicle populations, low yield, or dysfunctional exosomes.
The proper characterization of isolated particles is key to confirm an exosome phenotype. It should be noted that a small particle size is not exclusively indicative for exosomes, as same-sized non-exosome contaminants may contribute to this pool. Magnetic bead isolation improves the purification process by separating exosomes from other microvesicles based on the presence of specific surface markers. Yet, the non-reversible binding of the magnetic beads affect the integrity and function of exosomes.
The Fab-TACS® approach for traceless affinity exosome isolation is an affinity chromatography method based on our Strep-tag® technology. Low affinity Fab fragments (Fab-Streps) loaded into Strep-Tactin® agarose columns capture and release exosomes without affecting their functions. Based on the reversible binding principle of this method, the Fab fragments target the surface proteins of exosomes and spontaneously dissociate from the vesicles after the addition of biotin. Isolated exosomes are completely label-free and functional, and suitable for various downstream applications. A demonstration of Fab-TACS® for exosomes in comparison to other isolation methods is described in this application note: Isolation of exosomes from jurkat cells and human serum.
Surface-marker specific exosome isolation methods
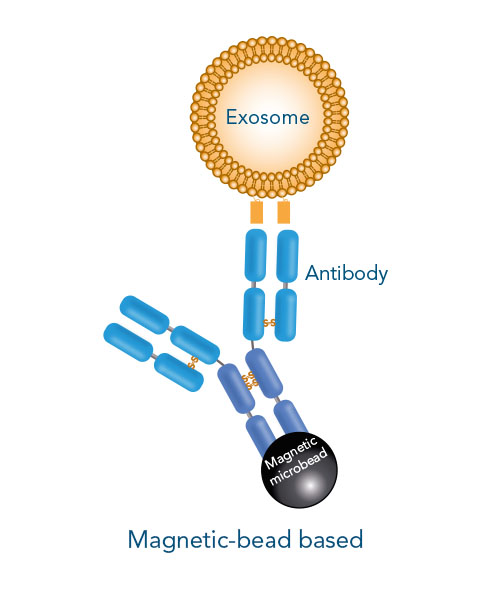
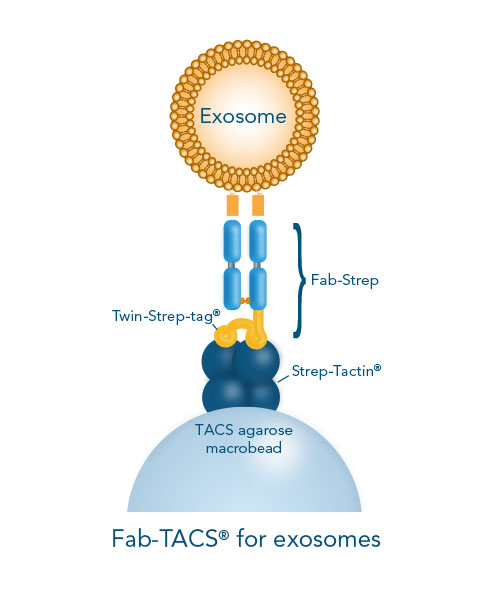
If surface marker-specific exosome isolation is required, high affinity antibodies conjugated to magnetic beads can be used. In contrast, Fab-TACS® for exosomes uses low affinity Fab fragments (Fab-Streps) in an affinity-chromatographic exosome isolation approach.