Immunostaining for protein detection
The term immunostaining covers a variety of methods for the specific detection and quantification of proteins from diverse samples, whereby “immuno” refers to the general use of antibodies. Immunostaining techniques include immunohisto- and immunocytochemistry flow cytometry, enzyme-linked immunosorbent assays (ELISA) and western blots.
These different methods are based on either colorimetric, chemiluminescence or fluorescence signals for detection. Besides the signal, the type of detection varies according to the experimental procedure. It can be chosen between direct detection, where a primary antibody carrying a label is used, and an indirect detection using an unlabeled primary combined with a labeled secondary antibody. As convenient alternative, we offer several solutions based on the Strep-tag® technology using Strep-Tactin® or Strep-Tactin®XT combined with Strep-tag®II or Twin-Strep-tag®.
Immunohistochemistry and immunocytochemistry
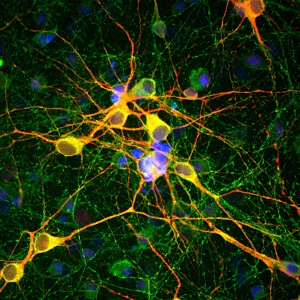
Amongst the immunostaining methods, immunohistochemistry is the most prominent one, in which whole tissues can be visualized, and thus, specific targets can be detected. In contrast to immunohistochemistry, in immunocytochemistry the focus is not on tissues but on cells. The advantage of both techniques is the possibility of imaging proteins in their proper histological context, which enables the localization of cellular compartments and can be detected using fluorescence microscopy. In both methods the sample fixation is essential to preserve the morphology and structure.
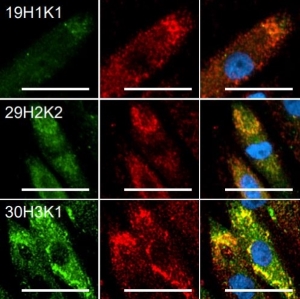
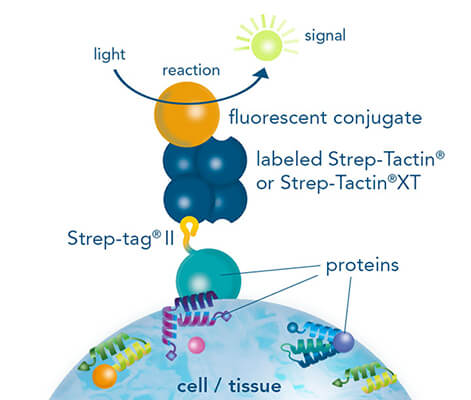
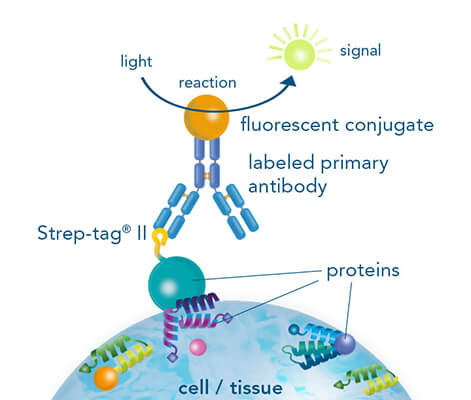
While both, chromogenic or fluorescent labels are utilized for detection, fluorescent labeling is especially popular due to the possibility of multicolor staining of different targets. Strep-tagged or biotinylated proteins, StrepMAB-Classic or StrepMAB-Immo conjugated to different dyes can be applied for direct target labeling. As viable alternative to antibodies, Strep-Tactin® and Strep-Tactin® XT with different fluorescent conjugates can be used as well. Due to their strong and highly specific interaction with Strep-tagged targets, both generate signals with high sensitivity, avoiding the need for a secondary antibody. However, when only small amounts of Strep-tagged or biotinylated proteins are present, the unconjugated murine StrepMAB-Classic can be used combined with a secondary antibody of choice.
Flow cytometry
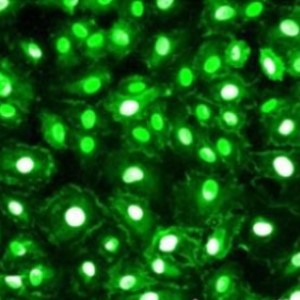
Fluorescently stained cells are frequently deployed in flow cytometry analysis, which can be conducted both, before and after cell isolation, delivering insights into cell functions for basic research and clinical trials. Here, the labeled cells pass a light beam inside a flow cytometer with high velocity, thereby providing a multitude of information on physical and chemical properties and a lucid quantitative display of different cell populations. Fluorescence-activated cell sorting (FACS) using a sorter is a specialized form of flow cytometry, where target cells are not subsequently discarded, but rather rigorously divided into defined subpopulations according to their fluorophore label – hence, surface marker identities – and retained for further downstream processing.
For staining of Strep-tagged targets, Strep-Tactin® or Strep-Tactin®XT conjugated to different fluorophores can be used or alternatively, the StrepMAB-Classic or StrepMAB-Immo antibody with different fluorescent conjugates.
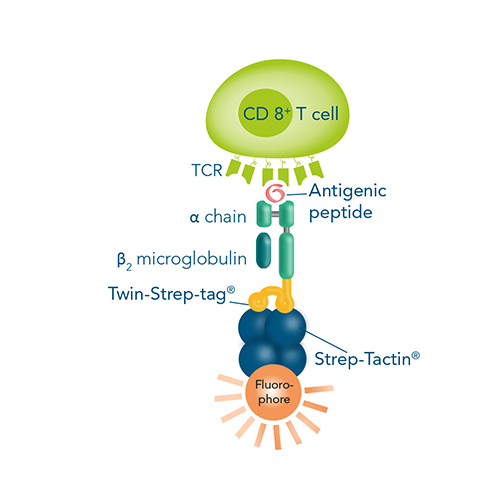
Besides the direct staining of the target, T cells represent a prominent target of this staining procedure due to their significant roles in immune response, and can be labeled in a surface marker- or antigen-specific manner for further investigation. While high-affinity antibody conjugates targeting individual T cell surface proteins are the most widely used tool, antigen-specific labeling can be achieved by the utilization of multimerized major histocompatibility complexes (MHCs) presenting the respective antigen.
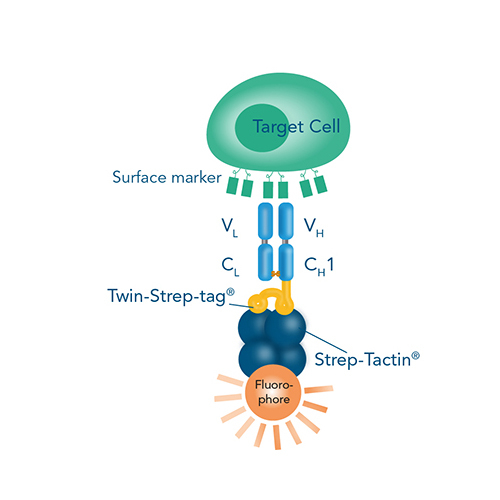
However, a major limitation of conventional cell labeling techniques applied for FACS is that the antibodies or MHC multimers used irreversibly bind the cells, block the epitopes and therefore possibly interfere with proper cell function. Our Fab-TACS®/Nano-TACS® (Traceless Affinity Cell Selection) and MHC I Streptamer® approaches enable fluorescent cell staining and isolation based on either recombinantly expressed MHC class I molecules with different peptides (MHC I-Streps) or on Fab-Streps/Nano-Streps. A special advantage of these techniques is the binding reversibility when combining these products with our Strep-Tactin® conjugates. This offers the possibility to remove all staining reagents after cell sorting and consequently to obtain highly pure and unmanipulated T cells.
Fab-TACS® and MHC I Streptamer® cell staining
Cell staining is possible using either Fab-Streps (or Nano-Streps) for surface marker-specific cell staining or MHC I-Streps for antigen-specific cell staining in combination with fluorescent Strep-Tactin® molecules.
ELISA – Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay, short ELISA, is a commonly used method for detection and quantification of ligands, such as proteins, peptides, or hormones even in a complex mixture. ELISA is a plate-based technique in which, depending on the type, either the target ligand or the capturing reagent is immobilized on a microplate. While there are different types of ELISA, the essential components are always consistent. Firstly, a ligand of interest, which is to be detected and potentially quantified. Secondly, an antibody, like StrepMAB-Immo or StrepMAB-Classic, or other detection reagent, like our conjugated Strep-Tactin®XT for specific recognition of the ligand. Lastly, an enzymatic reporter, the substrate conversion of which can be used as measurable signal.
While different formats exist, the basic principle when performing any kind of ELISA is the same.
- Coating or capturing of the directly or indirectly immobilized ligand on the microplate.
- Blocking of free surface-binding sites of the microplate.
- Detection of the ligand by using ligand-specific reagents, e.g. antibodies.
- Readout of signal produced by the enzymatic reaction of labeled primary or secondary antibody.
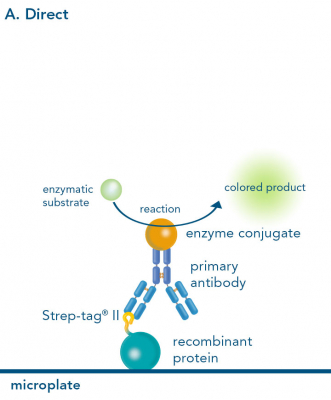
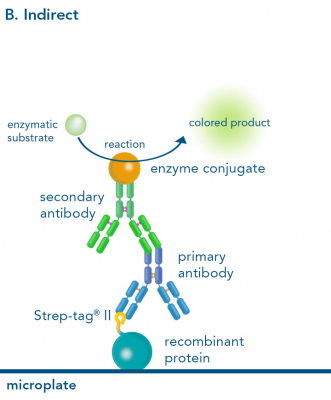
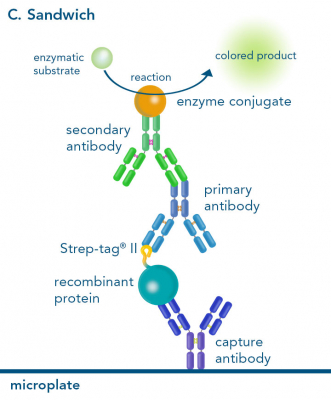
Depending on the experimental needs, one can choose between direct, indirect and sandwich ELISA. The formats differ in the way of capturing and detecting the target ligand.
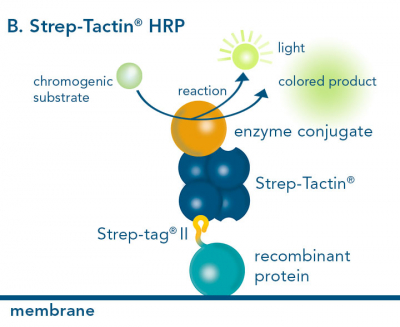
The direct and indirect ELISA both start with the immobilization of the ligand on a microplate. Whereas the direct ELISA only requires a labeled primary antibody for detection, in an indirect ELISA an unlabeled primary antibody first, and then a labeled secondary antibody is used. However, instead of a primary antibody it is possible to use Strep-Tactin® or Strep-Tactin®XT conjugates for Strep-tagged ligands, which is particularly beneficial because a second antibody for sensitive signal detection becomes redundant. Alternatively, StrepMAB-Classic conjugated with HRP can be applied, also avoiding the use of a secondary antibody.
In a sandwich ELISA, the plate is coated with a capturing molecule, like an antibody. Subsequently, the ligand is added and “sandwiched” between the capturing and the detecting antibody. Here, the primary antibody detecting the ligand is generally unlabeled, while a labeled secondary antibody is added for quantification. For capturing a desired Strep-tagged protein, plates can be coated using our StrepMAB-Immo or StrepMAB-Classic antibody. However, we recommend StrepMAB-Immo for this purpose due to its higher affinity towards the Strep-tag®. Convenient pre-coated plates with Strep-Tactin® or Strep-Tactin®XT are additionally available.
As enzymatic reporter, horse-radish peroxidase (HRP) is most commonly applied. It convert a certain substrate into a detectable product. As substrate, a variety of different chromogenic, fluorescent, and chemiluminescent products are available, always depending on the desired sensitivity and the laboratory equipment at-hand. In case a fluorescent detection method is desired, we offer various fluorescent labels conjugated to Strep-Tactin®XT, StrepMAB-Immo or StrepMAB-Classic.
Western blot
The western blot technique is an expedient method for the identification of specific proteins from a complex mixture. After extraction of the protein mixture, e.g. via cell lysis, the first step is to separate the proteins based on their molecular weight using gel electrophoresis. For protein separation by size, usually a polyacrylamide gel is prepared. Next, the proteins are transferred or blotted from the gel to a solid support, generally a nitrocellulose or polyvinylidene difluoride (PVDF) membrane. Because of its efficiency and speed, electrophoretic transfer is the most common blotting procedure. The gel and the membrane are “sandwiched” between two electrodes and the proteins are transferred due to their electrophoretic mobility, influenced by factors such as charge and size.
Since the membranes have a high affinity for proteins, the remaining binding sites of the surface need to be blocked after the transfer to prevent unspecific binding of the detection antibodies. A variety of different buffers and reagents is used in the whole process and, to minimize the signal-to-noise ratio, sufficient washing is required as intermediate step.
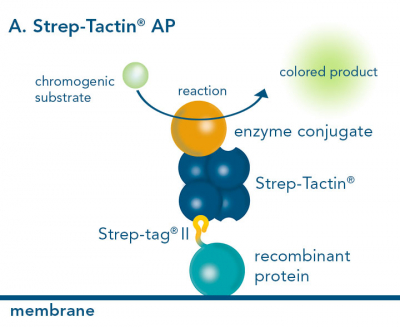
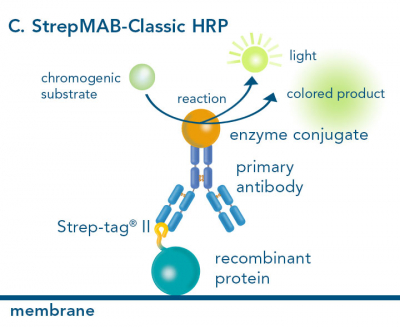
For detection of the antigen, it can be chosen between direct or indirect detection. For direct detection of Strep-tagged or biotinylated proteins, only a single labeled and target-specific antibody, such as our StrepMAB-Classic conjugated to HRP, is required. While it is quite common to use antibodies for detection, our Strep-Tactin® products conjugated to HRP or AP represent an equally beneficial alternative. These products are based on our Strep-tag® technology.
The combination of a primary antibody detecting the target and a labeled secondary antibody is widely used as well, as the second antibody leads to a signal amplification and provides different options for multiple detection methods. The choice of primary and secondary antibody depends on various parameters, such as the origin of the target protein, the species of the primary antibody or the desired detection method. When working with Strep-tagged proteins, our murine StrepMAB-Classic antibody is a good choice as primary antibody.
Nowadays widely applied labels are fluorophores or enzymes. As in the case of ELISA, horse-radish peroxidase (HRP) or alkaline phosphatase (AP) are the most common enzymes. Even though enzymatic labels require extra steps and usually have to be optimized, they are used most extensively due to the high sensitivity and the flexibility in detection when choosing between chromogenic, fluorogenic, and chemiluminescent substrates. When using fluorophores as labels, fewer steps are required, since no substrate development is necessary. For this purpose, we offer various fluorescent labels conjugated to Strep-Tactin®XT, StrepMAB-Immo or StrepMAB-Classic. The choice of the experimental procedure is generally dependent on the laboratory equipment, e.g., special devices for the detection of a fluorescent signal are needed.
Altogether, the relatively easy protocol for western blots offers a broad range of potential utilizations, such as detecting post-translational-modifications or verifying protein cloning, and makes it such a popular application.