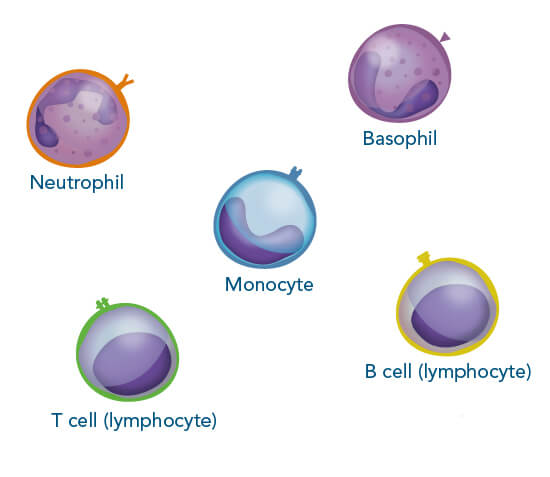
Cells serve as an important research tool to investigate different mechanisms in health and disease. They are also suitable for diagnostic and therapeutic purposes, making them attractive for a broad area of research fields. Immune cells have a wide range of functions such as controlling body homeostasis, which includes elimination of infected or cancerous tissue. Therefore, these cells are frequently employed for different analyses and model systems. The advantage of using immune cells is that they are abundant in, for example, human blood or mouse spleen, two sources that are relatively easily accessible for research purposes.
The interaction of cells with each other is very complex and for some experimental setups it is necessary to isolate one specific population for further downstream applications and analyses. Those include for example DNA/RNA isolation, single cell RNA sequencing, protein purification, Western blot or various cell culture experiments.
Positive and negative cell selection
Specific cells are most frequently isolated by targeting their surface markers or according to their antigen-specificity. For surface marker-specific cell isolation, generally one of these two approaches is used: positive or negative cell selection. Antigen-specific cell isolation usually requires a positive cell selection approach.

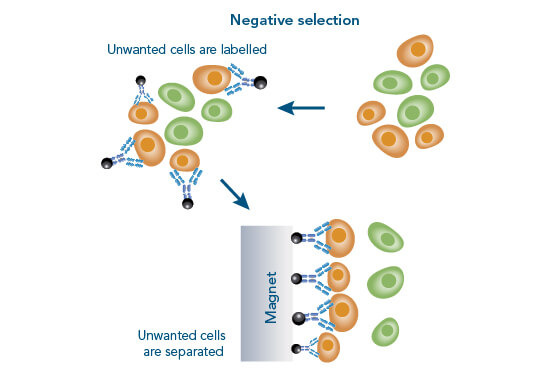
Cell isolation is possible via positive or negative cell selection. In positive cell selection, cells are directly labeled with e.g., magnetic bead-conjugated antibodies. In negative selection, all unwanted cells are labeled, leaving target cells completely “untouched”.
Surface marker-specific cell isolation
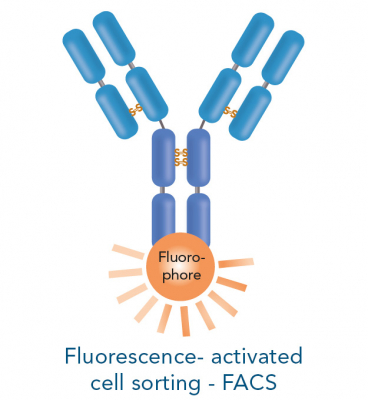
In positive selection, the target cells are directly labelled with for example antibodies conjugated to magnetic beads or fluorophores. The cells can subsequently be isolated using a magnet (magnetic-activated cell sorting - MACS) or a flow cytometer suitable for fluorescence-activated cell sorting (FACS).
Antibodies used for the positive cell selection approaches usually have a high affinity. This can cause unfavourable effects such as strong and almost irreversible binding to cells, cell stimulation (activation) as well as receptor blockade. In addition, magnetic beads often remain attached to the cells. Irreversible binding reagents can impair downstream applications due to a possible negative effect on cell functionality.
To avoid adverse effects of high affinity antibodies, Traceless Affinity Cell Selection (Fab-TACS®/Nano-TACS®) is the method of choice. This approach is based on our Strep-tag® technology and uses low affinity Fab-fragments or nanobodies (Fab-Streps/Nano-Streps) instead of high affinity antibodies. It is possible to choose between affinity chromatographic, magnetic and fluorescent isolation methods depending on the Strep-Tactin® backbone (Strep-Tactin® TACS Agarose columns, Strep-Tactin® Magnetic Microbeads or Strep-Tactin® PE/APC) and is therefore adaptable to different experimental requirements. All isolation methods result into completely label-free cells after the isolation.
Different methods for positive cell selection


Cell isolation using positive selection is possible via three principal approaches: fluorescence-activated cell sorting (FACS), magnetic-activated cell sorting (MACS) or traceless affinity cell selection (Fab-TACS®). In contrast to FACS and MACS that employ high affinity antibodies, Fab-TACS® uses low affinity Fab fragments.
In negative selection, the unwanted cells are labelled usually with antibodies conjugated to magnetic beads. The labelled cells are subsequently separated from the target cells using a magnet. To effectively use the negative selection approach, the composition of a sample has to be known to target all unwanted cells. Therefore, a standard problem of this method is limited purity and recovery, which causes variable quality. The advantage of this method is that cells remain completely “untouched” during the isolation procedure. However, highly pure populations can best be obtained by using positive separation techniques.
In this application note we demonstrate that the Fab-TACS® positive cell isolation method is also applicable for negative cell selection.
Antigen-specific cell isolation
Cells can also be isolated according to their antigen specificity. Instead of high affinity antibodies, major histocompatibility complexes (MHCs) that present a specific peptide/antigen are used to target T cells of interest. As a monomer, a stable binding to the cell surface is not possible and therefore MHC complexes need to be multimerized on a backbone to efficiently capture cells. Using backbones conjugated to magnetic microbeads or fluorophores also allows magnetic-activated as well as fluorescence-activated cell sorting in this system. Commonly used multimers are for example MHC Tetramers, MHC Pentamers or MHC Dextramers. However, these MHC multimers bind irreversibly to the T cell receptor, potentially affecting the original state of the cell.
Our Strep-tag® technology provides the MHC I Streptamer® approach that offers the possibility to remove all labelling reagents from the cell. In this approach, MHC I-Streps are multimerized on different Strep-Tactin® backbones (for example on fluorescent Strep-Tactin® or Strep-Tactin® Magnetic Microbeads) The multimerization is dissolvable, which subsequently induces the dissociation of resulting monomeric MHC I complexes from the cell surface.
The reversible labelling principle that is applied in all our isolation approaches helps to preserve the authentic properties, full effector function as well as viability of the target cells.