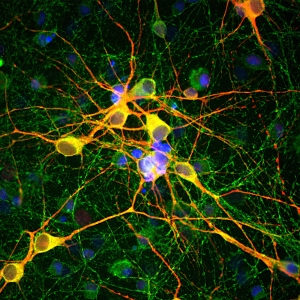
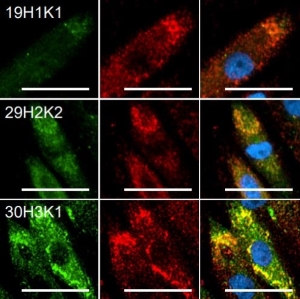
The Strep-tag® system enables cloning, expression, detection, purification, and immobilization of recombinant proteins. The highly specific interaction of the Strep-tag®II with Strep-Tactin® ensures efficient one-step purification of the protein of interest in unparalleled purity even from crude cell lysates. The mild and physiological conditions promote the yield of fully functional proteins, making the system particularly suitable for purification of enzymes as well as structural investigations, protein-protein interaction studies, ligand-receptor investigations, or even separation of living cells for re-culturing processes.
The Strep-tag® technology is compatible with a large number of protein classes, e.g. metalloproteins, membrane proteins, and fragile protein complexes with multiple subunits. Simultaneously, a high tolerance towards different buffers and additives promotes its universal applicability. The near covalent affinity (pM range) of Twin-Strep-tag® to Strep-Tactin®XT expands the range of applications of the Strep-tag® system towards protein analysis e. g. SPR analysis or bio-layer interferometry (BLI).
One tag - Multiple applications

The Strep-tag® protein purification system comprises two affinity tags, the 8AA Strep-tag®II and its tandem version Twin-Strep-tag®. Both versions can bind to Strep-Tactin® and its high affinity variant Strep-Tactin® XT. Thereby the two tags differ in the affinities with which they bind to Strep-Tactin® and Strep-Tactin®XT. Depending on the application and properties of the protein of interest one can combine the different tags and Strep-Tactin® variants according to the required affinity.
It is well known for its outstanding performance with regard to purifying recombinant proteins in a simple procedure with highest purities. Its latest development - the Strep-Tactin®XT - provides a binding affinity in low pM ranges in combination with Twin-Strep-tag® still maintaining binding reversibility and mild recovery of immobilized proteins. This improvement in affinity allows protein purification at high yields and purity, even for challenging proteins and from mammalian expression systems (e.g. Expi). Read more about the benefits of Strep-tag® purification in combination with high density mammalian protein expression in our white paper.
Furthermore, it fulfills the high demands of protein interaction analysis or assays and downstream applications like SPR, thus covering all steps from purification to immobilization efficiently.
We offer specialized matrices for any demand in form of ready to use kits, cartridges, gravity flow or spin columns, suspensions, coated microplates, magentic beads, and more (find products in our web shop). Additionally, we provide innovative solutions for approaches surrounding batch purification, FPLC/HPLC, high-throughput screens and assay development.
Strep-tag® protein purification system at a glance
Key features:
- Highly selective binding properties leading to unparalleled protein purity (> 95 %)
- Bioactive target proteins due to the rapid one-step purification under target specific conditions
- Variability in buffer conditions, e.g. high salts, detergents, metal ions, chelators or reducing agents
- Peptide tag with balanced/inert amino acid composition and, therefore, the protein structure or activity is not influenced, and removal of tag is not required
- Favorable for protein:protein interaction studies due to mild elution conditions and low washing volumes
- Universal toolbox including products for cloning, purification, and analytic applications (ELISA, FACS, SPR, Microscopy, Western Blot, etc.)
When should the Strep-tag® purification system be the method of choice?
Besides the purification of classical proteins the Strep-tag® system should be the method of choice for:
- metalloproteins
- membrane proteins
- low abundant proteins (in combination with Strep-Tactin®XT)
- sensitive protein complexes with multiple subunits
- bioactive proteins
Is There An Alternative To His-tag?
Choosing an appropriate affinity chromatography system for simple and efficient protein purification is a common question. There are differences between the systems which rarely are clearly represented, making it hard for scientist to reach a decision.
This comprehensive comparison of the most frequently used systems, His-tag and Strep-tag®, explains these differences as well as recommends one system depending on the properties of the target protein, expression host and purification conditions.
Strep-tag® system - quick & easy protein purification protocol
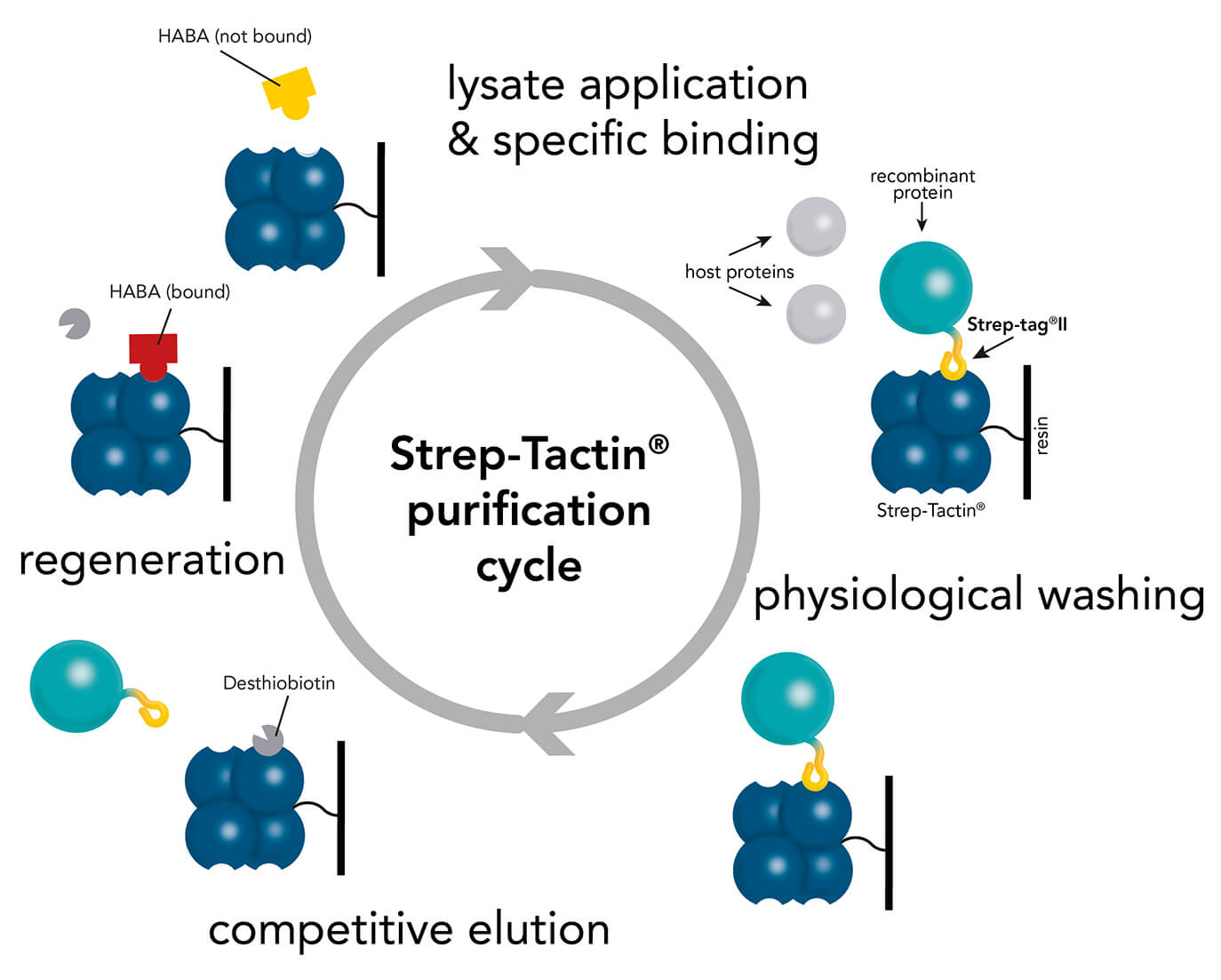
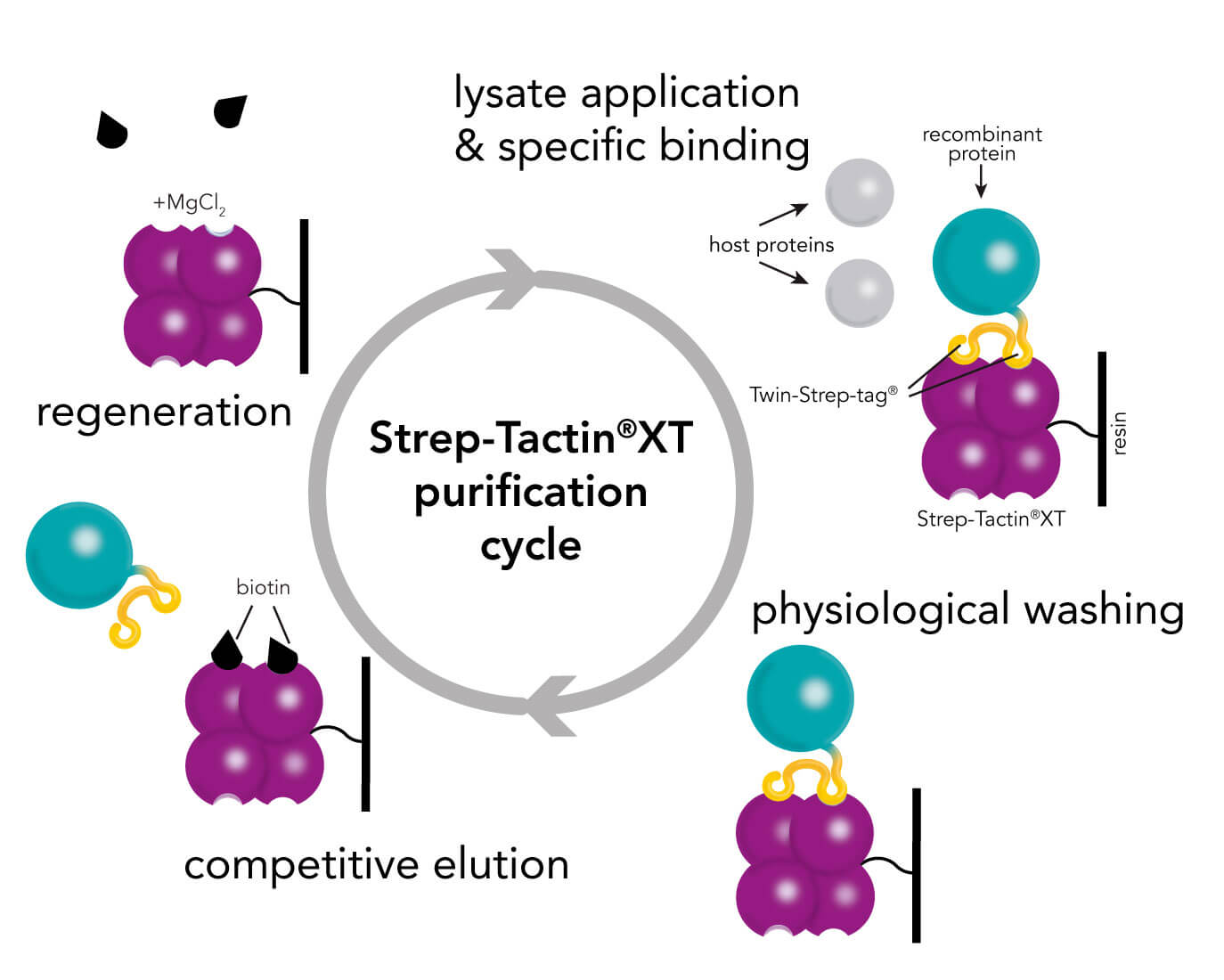
Strep-Tactin® and Strep-Tactin®XT purification cycle
For the purification of Strep-tag®II or Twin-Strep-tag® proteins both resin types, Strep-Tactin® and Strep-Tactin®XT, are applicable. Protein purification with Strep-Tactin® as well as Strep-Tactin®XT is very simple and fast, since the purification cycle for each target protein is the same. However, if a target protein needs a specific buffer composition, e.g. PBS, HEPES or no chelating agents, like EDTA, the buffers can be easily adapted without the need of changing the purification cycle. Both resin types accept various buffer compositions, reagents and additives and an overview is given in the compatible reagents list for Strep-Tactin® as well as Strep-Tactin®XT.
The purification cycles for both resin types are highly similar. However, the sample application, the elution, and the regeneration step are slightly different and will be explained in the following.
A comparison of purification via Strep-Tactin® and Strep-Tactin®XT depicts two changes in the procedure. The first and second step (lysate application & wash) remain the same. But the elution and the regeneration streps are different for both systems. For elution from Strep-Tactin® desthiobiotin is used whereas Strep-Tactin®XT requires biotin for elution. Also the regeneration step differs. HABA is used for regeneration from Strep-Tactin® and in case of Strep-Tactin®XT 3 M MgCl2 is applied.

Properties of Strep-Tactin® and Strep-Tactin®XT
The shared benefits of Strep-Tactin® and Strep-Tactin®XT are
- Purification of bioactive recombinant proteins under physiological conditions
- Protein aggregation is avoided
- Broad range of detergents, chelators, salt or redox conditions allowed
- Highly specific interaction of Strep-tag® with Strep-Tactin®, no unspecific protein binding (high specificity in screening and assays)
Additional benefits of Strep-Tactin®XT due to increased binding affinity in pM range:
- Efficient, directed, and reliable immobilization of targets from complex mixtures while maintaining the reversibility
- Allows all kinds of analytic applications as e.g. surface plasmon resonance (SPR) or biolayer interferometry (BLI)
Furthermore, higher protein yields compared to Strep-Tactin® are obtained. On average, StrepTactin®XT provides almost 2-fold more protein than Strep-Tactin®.
Strep-Tactin®XT also ensures sharp elution profiles achieving high concentration of the target protein. Strep-Tactin®XT even tolerates a wide pH range in addition to various detergents and additives, contributing to the universal usability of the system.
Resin re-use
Strep-Tactin® and Strep-Tactin®XT resins for protein purification can be regenerated and re-used 3 to 5 times without loss in performance. The proper regeneration of the column and resin activation can easily be checked with HABA. The yellow HABA solution turns red (Strep-Tactin®) or orange (Strep-Tactin®XT) upon binding to the engineered biotin binding pockets of Strep-Tactin® and Strep-Tactin®XT clearly indicating that the resin is fully regenerated. Afterwards, HABA can be removed by washing with 1x Buffer W. Once the red color has disappeared the column can be re-used. If the biotin binding pocket is blocked or damaged no color shift occurs and the resin cannot be re-used.
Specifications for Strep-Tactin® Purification Resins
There are several Strep-Tactin® resin versions available. All resins differ in their properties and suitability for applications. Learn more about the different resin matrices in order to know when to use which resin.
Strep-Tactin® | Strep-Tactin®XT | ||||
| Matrix | Superflow® | Sepharose® | MacroPrep® | 4Flow® | MagStrep "type3" XT beads |
| only available as 20 ml slurry) | |||||
| Gravity flow column | yes | yes | yes | yes | no |
| Batch purification | no | no | no | yes | yes |
| FPLC/HPLC | yes | no | no | yes | no |
| Binding capacity* | classic: 3 mg/ml resin | classic: 3 mg/ml resin | classic: 3 mg/ml resin | classic: not determined | classic: 25.5 mg/ml resin |
| high capacity: not determined | high capacity: not available | high capacity: not available | high capacity: not determined | high capacity: not available | |
| Dynamic binding capacity** | classic: not determined | classic: not determined | classic: not determined | classic: 5 mg/ml resin | classic: not determined |
| high capacity: 7.0 mg/ml resin | high capacity: not available | high capacity: not available | high capacity: 14 mg/ml resin | high capacity: not available | |
| Bead structure | 6% agarose, crosslinked | 4% agarose, crosslinked | polymethacrylate | 4% agarose, highly crosslinked | magnetic beads covered with 6% agarose, crosslinked |
| Bead size | 60-160 µm, spherical | 45-165 µm | 50 µm | 50-150 µm, spherical | 25 μm (average), spherical |
| Exclusion limit | 6 x 106 Da | ~3 x 107 Da | 1 x 106 Da | 3 x 107 Da | not specified |
| Recommended flow rate | 0.5-1 ml/min | not specified | 0.5-1 ml/min | 0.5-1 ml/min | not specified |
| pH range for protein binding | 7-8 | 7-8 | 7-8 | 4-10 | 6-10 |
| Max pressure | 9.6 bar | gravity flow | 70 bar | 9.6 bar | not specified |
| Storage | 4 °C, do not freeze | 4 °C, do not freeze | 4 °C, do not freeze | 4 °C, do not freeze | 4 °C, do not freeze |
| Shipment | RT | RT | RT | RT | RT |
| Eluent | desthiobiotin | desthiobiotin | desthiobiotin | biotin | biotin |
| Regeneration buffer | Buffer R | Buffer R | Buffer R | Buffer XT-R | Buffer XT-R |
| Features | The Strep-Tactin® Superflow high capacity binds in comparison to Strep-Tactin® Superflow a three to five fold higher amount of target protein and thereby achieves a higher yield. | Strep-Tactin® Sepharose is a good alternative for large proteins, if the affinity of Strep-Tactin®XT 4Flow® is too high. | The alternative when cell components (e.g. chlorophyll from plant extracts) bind non-specifically to Sepharose, Superflow or 4Flow®. | Strep-Tactin®XT 4Flow® is the resin to start with. Due to the higher affinity compared to Strep-Tactin® resins it is particularly applicable for low abundant or challenging proteins. | MagStrep "type3" XT beads are perfectly suitable for batch and small-scale purifications. Due to the magnetic core the beads can be easily separated with a magnetic device without lossing the sample during the pipetting steps. |
| If the affinity of Strep-Tactin®XT resins is too high and the target protein is not fully eluted, then Strep-Tactin® Superflow is a good alternative. | Only suitable for gravity flow chromatography due to the low pressure stability. | Synthetic support in comparison to all other resins. | Suitable for all types of proteins and especially for large proteins, because it has the high affinity of Strep-Tactin®XT, the broadest pH stability and consists of a low-concentrated agarose. | Suitable for well expressed proteins (> 1 mg/ml). | |
* Max binding capacity for a Strep-tag® protein (30kDa). ** Dynamic binding capacity (DBC) was determined with mCherry-Twin-Strep-tag (30 kDa) under realistic operation conditions and shows the amount of protein which is bound until 10% of the protein is found in the flow through. | |||||